At present, although liquid electrolytes are widely used, solid electrolytes have gradually attracted widespread attention. If solid electrolytes completely replace liquid electrolytes, all-solid-state ionic devices can be obtained, which will also be a major change in the field of electrochemical device energy storage. . Generally speaking, the room temperature conductivity of a solid electrolyte with practical value should be in the range of 1 to 10^-5S·cm-1. This value is between metals and insulators, and is comparable in magnitude to the ionic conductivity of semiconductors and liquid electrolytes, as shown in Figure 8-1.
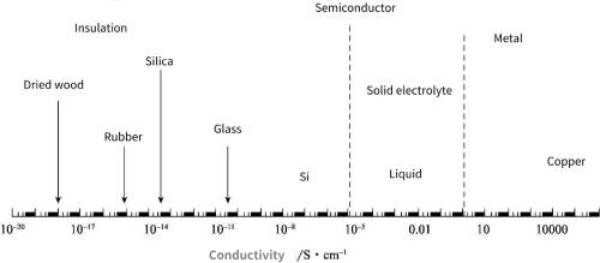
Figure 8-1 Comparison of room temperature conductivity of solid electrolytes and typical metals, semiconductors and insulators
Note: The conductivity range of the solid electrolyte is shown by the dotted line in the figure
Solid-state electrolytes can also be called “superionic conductors” or “fast ion conductors”, which are materials that have an ionic conductivity comparable to that of molten salts or liquid electrolytes in their solid state. An ideal inorganic solid electrolytes material needs to meet the following requirements:
①It must have good ionic conductivity (10-1~10^-4S cm-1) at working temperature;
②It has extremely low electronic conductivity (<10^-6S cm-1);
③ The chemical stability is better, and it cannot chemically react with the electrode material;
④ has a small grain boundary resistance;
⑤ The coefficient of thermal expansion matches the electrode material;
⑥High electrochemical decomposition voltage (>5.0V);
⑦Environmentally friendly, cheap and easy to obtain raw materials, easy to prepare, etc.
The interior of solid electrolytes contains many defects, such as ionic vacancies and interstitials. Its structure and properties are between normal crystals (with regular three-dimensional structure and immovable atoms and ions) and liquid electrolytes (without regular structure, but ions can move freely), which is very suitable for ion migration. favorable. Its structure is related to its ion conduction mechanism, electrochemical properties, etc., and has attracted widespread attention in the fields of physics and electrochemistry, and has attracted a large number of workers to develop and study it.
For the current solid electrolytes, we can roughly divide them into three categories:
①Inorganic solid electrolytes (ISEs);
② Solid polymer electrolytes (SPEs);
③ Composite solid electrolytes (CSEs). The composition of inorganic solid electrolytes is diverse, and can be subdivided into oxide, sulfide, and nitride solid electrolytes according to the different heterocyclic atoms on their ligands. Figure 8-2 shows some applications and possible potential applications of solid electrolytes.
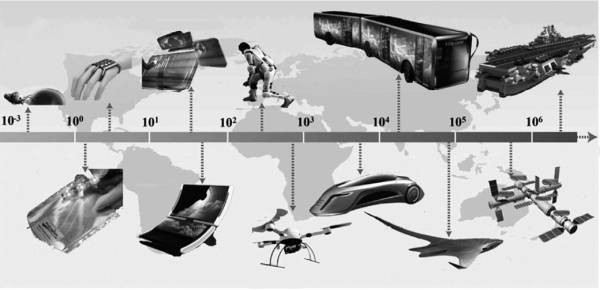
Figure 8-2 Application of solid electrolyte
Ion transport in solid electrolytes is different from the coupled transport of cations and anions in liquid electrolytes. However, due to the inherent high conductivity of inorganic solid electrolytes (ISEs), under certain conditions, their conductivity will increase with the rise of temperature. When the temperature rises from room temperature to 300 °C, the conductivity of typical solid electrolytes is generally It can be improved by 2 to 3 orders of magnitude.
From 1854 to 1888, Buff and others studied the conductivity of glassy and crystalline silver halide (AgBr, AgI), measured their thermodynamic and kinetic parameters, and studied their ion migration. At the end of the 19th century, solid electrolytes were first applied to the preparation of oxygen ion conductors. Oxygen ion conductors were used as light sources for incandescent lamps. When current passed through the electrolyte, the resistance decreased and light was emitted. In 1904, the solid electrolyte was proved to be applicable to Faraday’s law. At the same time, the discovery of silver iodide, a low-temperature solid electrolyte material, has ended people’s misunderstanding of superionic conductive solid materials, and is an important breakthrough in the research process of solid electrolytes.
By the middle and late 20th century, lithium-ion conductors gradually began to develop. In the mixed system of LiI and Al2O3 (pore size 3-15nm), it is found that the room temperature conductivity of lithium ions in this system is as high as 10-4S cm-1, which is increased compared with the composite material mixed with Al2O3 particles with a particle size of 10μm. 1 to 2 orders of magnitude, interface stability increased by 50%. For this phenomenon, the researchers speculate that the improvement of its performance is mainly due to the fact that Li+ is adsorbed on the surface of Al2O3 with nucleophilicity, forming a space charge region and thus improving the conductivity of ions. Energy, electricity, etc. are closely related to alkali metal ion conductors. Many researchers have been engaged in the research and development of this type of conductors, which has played a positive role in promoting the development of solid electrolyte materials.
8.1 Inorganic Solid Electrolytes
Since most inorganic solid-state electrolyte materials have a special crystal structure and anisotropic electrical conductivity, this enables the rapid transport of ions in the gaps of the framework structure. To design a crystalline inorganic ionic conductor, it needs to meet the following basic criteria: First, the conductor should contain disordered mobile ions of a size suitable for the smallest cross-sectional area in the conduction channel, with different coordination numbers The stable framework ions, the force between the mobile ions and the main framework is weak.
The research on inorganic solid-state electrolytes began with LiI, Li3N and their derivatives. Although the room temperature ionic conductivity of Li3N is as high as 6.0×10-3^S cm-1, its electrochemical decomposition voltage is only 0.45V. its application in practice.
The most widely studied inorganic solid electrolytes are LIPON type, NASCION type, perovskite type, sulfide, Garnet and LiN3 types. The ionic conductivity of LIPON electrolyte is low at room temperature, and most of the manufacturing process needs to be completed under vacuum conditions, so it is difficult to produce on a large scale, the cost is high, and it is difficult to be widely used; Ti4+ exists in NASCION and perovskite electrolytes, When in contact with lithium metal, oxidation-reduction reactions are prone to occur, and the electronic conductivity is high, and there is a large grain boundary resistance; the sulfide electrolyte has high ion conductivity, but poor thermal stability, strong hygroscopicity, and contact with water It is easy to generate toxic hydrogen sulfide gas when it is used; the conductivity of LiN3 type electrolyte is anisotropic, poor chemical stability, easy to form heterogeneous phases during the synthesis process, and flammable when exposed to water, which limits its commercial application.
Inorganic solid electrolytes were first used in the preparation of high energy density power batteries. The sodium-sulfur battery invented by Ford in 1976 has the structure: (-)/Na (liquid)/Na-β-Al2O3/S (Na2Sx) liquid/(+). The theoretical energy is 780W·h·kg-1, generally 150~300W·h·kg-1, while the theoretical value of lead-acid battery is 180W·h·kg-1, actually only 30~50W·h·kg-1 .
Devices such as all-solid-state batteries, solid-state fuel cells, and all solid-state capacitors require absolute high safety. Using inorganic solid electrolytes to replace traditional liquid electrolytes is one of the feasible ways to solve the safety problem of secondary batteries. In order to develop all-solid-state batteries, in addition to solving the contact problem between the interfaces, it is more critical to find a solid electrolytes with high ionic conductivity.
The research team of Tokyo Institute of Technology, Toyota Motor Corporation and High Energy Accelerator Research Institute recently developed a superionic conductor with the highest lithium ion conductivity. The lithium ion conductivity of this superionic conductor (Li10-GeP2S12) is at room temperature It can reach 12mS·cm-1, which is not only twice the conductivity of the previous lithium ion conductor Li3N (6mS·cm-1), but also much higher than the value of the ionic conductivity of the colloidal electrolyte. In addition, the silver-ion battery with solid electrolytes Ag4RbI5 as the diaphragm has been widely used abroad due to its good battery performance, but the disadvantage is that the cost is very high.
At present, the most promising sodium-ion solid electrolytes are Na-β″-Al2O3 and NASICON-structured sodium-ion electrolytes. Among them, the Na-β″-Al2O3 electrolytes that have been commercialized have high ionic conductivity. The β″-Al2O3 material is a layered structure, the transport of Na+ in Na-β″-Al2O3 is two-dimensional conduction, its conductivity is directional, and the anisotropic thermal expansion coefficient will reduce its service life, and Its sintering temperature is as high as 1680 °C, which is not easy to process and manufacture. It needs to be carried out in a closed container to prevent the volatilization of Na2O during sintering, and the anisotropic thermal expansion during thermal cycle may reduce the life and moisture resistance of the inorganic ceramic film, and the production cost Higher, so in order to overcome the above shortcomings, people are working hard to explore new sodium ion conductors.
Inorganic solid electrolytes are widely used in dye-sensitized solar cells. In 1995, Tennakone et al. assembled dye-sensitized solar cells (DSSCs) for the first time using CuI as a solid electrolytes. Its current density is about 2.5mA/cm-2 measured under sunlight (about 800W·m-2), but due to the difficulty in controlling the grain size of CuI, it cannot be well combined with TiO2, so its photoelectric efficiency and stability It has been affected to a certain extent, and it was found in the experiment that the current began to attenuate after 1.5 to 2 hours of continuous operation.
Meng et al. used ZnO with better conductivity to improve the electron transport between TiO2 particles, and used 1-methyl-3-ethylimidazole thiocyanate to control the growth process of CuI particles. The solid-state dye assembled by this method The photoelectric conversion efficiency of the sensitized solar cell is 3.8%.
The dye-sensitized solar cell obtained by O’Regan et al. using CuSCN as a solid electrolytes has a current density of 8mA cm-2, an open circuit voltage of 600mV, and a photoelectric conversion efficiency of about 2% under a light intensity of 100mW cm2. The application of inorganic solid electrolytes in dye-sensitized solar cells still faces many problems to be solved, such as improving the hole transport efficiency and electrolyte stability, solving the problem of combining electrolytes with nanocrystalline films, electrolyte conduction bands and sensitizing dyes. The problem of collocation selection and the preparation of n-type nanoelectrodes.
For electric double-layer supercapacitors, traditional supercapacitors can be roughly divided into two types according to different assembly methods. One is a reel type capacitor. The electroactive material is coated on the current collector and assembled by winding. , which is characterized by being able to accommodate large-sized electrode sheets, and has a high total capacitance; the other type is button capacitors, which assemble disc-shaped or flat-shaped electrode sheets by stacking sandwiches, and are characterized by packaging density. High, easy to connect in series and parallel, but the monomer voltage is low. The electrolyte of traditional supercapacitors is generally liquid or organic electrolyte, which is easy to leak, has high requirements for packaging, and has certain potential safety hazards. The new type of solid-state supercapacitor has no danger of electrolyte leakage because its electrolyte is solid, the difficulty of packaging is reduced, the stability is improved, and it is smaller and more flexible than traditional supercapacitors. High safety, wide potential window, easy processing and packaging are one of the development directions of supercapacitors in the future. However, inorganic solid electrolytes are limited by the conduction and movement of active materials, so their applications in supercapacitors are currently rare.
8.2 Solid Polymer Electrolyte
Polymer electrolytes (polymer electrolytes) are broadly defined as electrolytes that contain polymer materials and can conduct electricity like liquids, and refer to substances with ionizable groups on the polymer chain. For a while it was thought that polymers were insulators and did not conduct electricity. Until 2000, a researcher made an epoch-making discovery of the conductivity of plastics, breaking the traditional concept that polymers do not conduct electricity, and creating a new field. Since then, the research on polymer electrolytes has been carried out rapidly all over the world. The research contents mainly include the synthesis of new polymers, the research on the physical and chemical properties of polymers and polymer electrolytes, and the establishment of theoretical models for charge transport.
Pure solid polymer electrolytes are the earliest researched polymer electrolytes. Polymers can be divided into three classes: crystalline polymers, semi-crystalline polymers and amorphous polymers. However, whether crystalline polymers or amorphous polymers can better assist ion transport has not been accurately determined. The study of microstructural dynamics in polymer matrices can better understand the mechanism of ion migration to optimize the performance of solid-state polyelectrolytes (SPEs). Therefore, with the development of related testing instruments, many in-depth studies have begun to focus on the ion migration mechanism.
So far, there are many ways to classify polymer electrolytes. According to the polymer matrix, they can be divided into five categories: polyethylene oxide (PEO), polymethyl methacrylate (PMMA), polybiased Vinyl fluoride (PVDF), polyacrylonitrile (PAN), polyvinyl chloride (PVC) polymer electrolytes. According to the shape of the polymer, the polymer electrolyte can be divided into solid polymer electrolyte (solid polymer electrolyte, SPE), gel polymer electrolyte (gel polymer electrolyte, GPE), composite polymer electrolyte (composite polymer electrolytes, CPE), etc. In addition, polymer materials containing -NH-, -CN-, CC-OH and other functional groups in the polymer molecular chain can also be used as the matrix material of solid polyelectrolyte (SPE). This polymer electrolyte can be regarded as a solid solution (salt-in-polymer) formed by dissolving the electrolyte salt in the polymer matrix.
8.2.1 Polyethylene oxide (PEO)
Among solid polyelectrolytes, polyethylene oxide (PEO) solid polyelectrolytes have been widely used due to their mature research techniques and excellent properties. PEO is a high-molecular-weight homopolymer formed by heterogeneous ring-opening of ethylene oxide, and is the simplest skeleton structure system. The dissolution effect of PEO is determined by its unique molecular structure and spatial structure. It can not only provide a sufficiently high electron-donating group, but also has a flexible polyether chain segment, so it can effectively dissolve cations with a cage effect. In 1973, Wright et al. reported for the first time that ions in polymers can also migrate, that is, the SPE system of PEO alkali metal salt has ion conductivity. On the basis of previous work, Armand et al. applied PEO and alkali metal salt system to battery research. PEO polymer and lithium salt can form a stable complex system, which is beneficial to the dissociation of lithium salt. PEO is a semi-crystalline polymer with 80% crystallinity, low ion conductivity at room temperature (σ<10-5S cm-1), and can only operate normally above 60°C. In 1975, Wright et al. found that the alkali metal salt complexes of polymers such as polyethylene oxide (PEO) and polyvinylidene fluoride (PVDF) had ion conductivity, and made polyacrylonitrile (PAN) and polymethyl Methyl acrylate (PMMA) ion-conducting membrane.
8.2.2 Polyacrylonitrile (PAN)
Since 1975, researchers have gradually turned their attention to the research of polyacrylonitrile (PAN) series electrolytes. Due to the advantages of simple synthesis, good stability, high heat resistance, and non-flammability, PAN has received extensive attention. Due to its limited ionic conductivity, it is generally plasticized with an organic electrolyte to form a gel polymer electrolyte. The production method is generally to mix polyacrylonitrile powder with non-aqueous electrolyte solvent, heat to 100°C to dissolve, and then cool. Raghavan et al. used PAN as the matrix to make gel polymer electrolytes, and studied gel electrolytes containing different liquid electrolytes. The results showed that their conductivity at 25°C was in the range of 10-3S cm-1, The electrochemical window stability is greater than 4.7V, the charging/discharging stability is good, and has practical application value.
8.2.3 Polymethylmethacrylate (PMMA)
Polymethyl methacrylate (PMMA) is an amorphous polymer with good transparency. There is a carbonyl side group in the MMA unit, which has a strong effect on the oxygen in the carbonate plasticizer and can accommodate a large amount of liquid electrolyte. Therefore, PMMA has better ion transport performance, and its interface impedance with metal lithium electrodes is low, and it has better interface stability. It is an ideal gel-type polymer electrolyte framework component. PMMA is also rich in raw materials, easy to prepare, and cheap, so it is widely used. Since 1985, it has been used in lithium secondary batteries as the main body of polymers.
The affinity between the polymer and the solvent affects the solvent retention capacity, mechanical properties and electrical conductivity of the gel film. Polymers with strong affinity have high solvent retention capacity and high electrical conductivity, but their mechanical strength is poor. PMMA has a strong affinity, but its independent film-forming performance is poor. It can be combined with low-affinity polymers such as polyvinylidene fluoride (PVDF), polyvinyl chloride (PVC), polyvinylidene fluoride-hexafluoropropylene [P (VDF-HFP )], acrylonitrile (ABS), etc. can make the performance of the gel-type polymer electrolyte more excellent. When preparing MMA-styrene (ST) copolymers, when the molar ratio of MMA:ST is 33:67, the electrochemical performance of the system is the most stable, and the conductivity at room temperature exceeds 1.0×10-3S·cm-1, and the formation Good film.
8.2.4 Polyvinylidene fluoride (PVDF)
PVDF is composed of -CH2-CF2- repeating units and is a crystalline polymer with a crystallinity of 30% to 50%. Its structure is shown in Figure 8-3. PVDF has high crystallinity and poor mechanical properties. It is usually copolymerized with hexafluoropropylene to form a copolymer [P(VDF-HFP)] to reduce the crystallinity of the polymer, improve the electrical conductivity, and the melting point will also decrease. It also increases the flexibility of the polymer film.
Figure 8-3 PVDF structure
8.2.5 Polyionic Liquids
In order to make polymer electrolytes practically used in a wider field, the structure of ionic liquid (IL) can be introduced into polymer electrolytes, and the advantages of high conductivity of ionic liquids can be used to overcome the relatively low performance of polymer electrolytes. Defects in ionic conductivity. Ionic liquids usually have high viscosity, so they are not suitable as ideal liquid electrolytes. Additionally, there is a growing demand for non-liquid electrolytes due to possible battery leakage issues. ILs can be polymerized to form ionic polymers, which have somewhat similar ion mobility to ILs, and they are not liquid. Copolymer electrolytes formed on the basis of such polymers include copolymers of vinylidene fluoride and hexafluoropropylene [P(VDF-HFP)] and copolymers of acrylonitrile-methyl methacrylate-styrene [P(AN -MMA-ST)] and so on.
Polyionic liquid (PIL) is a polymeric ionic liquid, which is a polyelectrolyte composed of repeated ionic liquid monomers. From monomers to oligomers to polymers, some special properties of ionic liquids such as low vapor pressure, thermal stability, non-flammability, high ion conductivity and wide electrochemical window stability can transferred to the polymer chain. Furthermore, the designability of ionic liquids and the selectivity of polymer segments enriches the properties and applications of PILs, attracting considerable attention in the field of polymer and materials science.
In the early review of polyionic liquids, their synthesis, chemical structure, physical properties and applications in materials were systematically discussed. Polyionic liquids have developed rapidly in the past 5 years. A series of functional polyionic liquid materials can be synthesized by introducing the anions and cations of functional ionic liquids (such as imidazole, pyridine, pyrrole and hexafluorophosphate) into the polymer polymer system, which broadens the properties, structure, and functions and applications.
Integrating the conductivity, hydrophilicity, hydrophobicity, and thermodynamic stability of ionic liquids into polyionic liquids with adjustable structures has been used in innovative applications in different fields. From synthesis to application, it is critical to understand how to design a PIL to meet the desired performance criteria.
Ionic conductivity is often an important property to consider when designing PILs, especially when solid-state electrolytes are used in electrochemical and electrochemical devices. Unlike ionic liquids or ionic liquid polymers where both anions and cations are mobile, PILs are usually single-ion conductors. Usually the anion or cation of PIL is structurally constrained as part of the polymer backbone. After polymerization has occurred, the ionic conductivity typically decreases significantly compared to the monomeric ionic conductivity. This is due to a significant increase in glass transition temperature and a decrease in the number of mobile ions after covalent bonding of component ions. Some relevant factors may affect the ionic conductivity in PIL, such as glass transition temperature, polymer structure, molecular weight, and thermal stability of the polymer chain.
(1) Glass transition temperature (Tg)
A lower Tg increases ionic conductivity. Although the electrostatic interaction between electrostatic ion pairs is weak, PIL may exhibit a low Tg due to the high charge density. This effect is different from conventional ionic polymers that have high glass transition temperatures due to strong electrostatic interactions. The Elabd research group found that replacing B with TFSI- can enhance the ionic conductivity in PIL. A significant decrease in Tg facilitates partial movement of the polymer and increases anion mobility. The Tg size of polymers with poly(1-butyl-3-vinylbenzylimidazole) (P[VBBIM]) skeleton structure has the following order according to different anions: P[VBBIM][TFSI-] (3℃) <P[VBBIM][Sac](40℃)<P[VBBIM][BF4](78℃)<P[VBBIM][PF6](85℃). Among them, TFSI- and Sac-anions can significantly reduce the Tg of the polymer because they plasticize the bulk polymer. Similarly, Baker et al. directly connected TFSI- to the polyoxyethylene skeleton, which reduced the glass transition temperature of polyionic liquid (-14°C) and increased its conductivity (10-5S cm at 30°C -1).
(2) Polymer structure
A polymer with the same cation but a smaller anion has a higher conductivity than a polymer with a larger anion because the negative charge of the smaller anion moves faster. For various polymers with the same pyrrolidine cation but different anions, the conductivity is determined by the delocalization of the anionic charge and its size. The higher the degree of anion delocalization of S-containing polymers, the greater the conductivity. It is worth mentioning that among a series of strongly delocalized anions, polyelectrolytes with cyanosulfonimide ions exhibit excellent conductivity.
The substitution position of the imidazole cation and the type of anion have a significant impact on the ionic conductivity. Polycationic and polyanionic ionic liquids are flexible on vinyl and the conductivity of ILs of this type of structure is 10-4S·cm-1 at room temperature. It is necessary to maintain the flexibility of the imidazole cation to obtain higher ionic conductivity of IL-type polymers. Imidazole cation mobility, translation, and rotation all affect ionic conductivity.
(3) Molecular weight
The molecular weight of PIL can also affect its Tg, viscosity, modulus, and ionic conductivity. Experiments have found that the ionic conductivity of imidazolium polycations decreases with increasing molecular weight. Fan et al. explored a series of PILs with degrees of polymerization ranging from 1 to 333 and molecular weights from 482 to 160,400 Da. They found that as molecular weight increases, the ion transport mechanism shifts from tightly coupled to piecewise kinetics to strongly separated. The less likely integration of polymer cations may contribute to decoupling processes in ion transport. When the degree of polymerization n exceeds about 70, Tg increases with increasing molecular weight, similar to the behavior of conventional flexible uncharged polymers. The conductivity decreases sharply with increasing molecular weight (from monomer to trimer), but does not change significantly in the high molecular weight region (above 1000 Da).
(4) Thermal stability
Research on the thermal stability of PIL is necessary because in many cases polymers will be used in high-temperature fields. The diversity of PIL structures also leads to a wide thermal stability range of PIL, from 150°C to over 400°C. In thermogravimetric analysis experiments, the setting of the initial decomposition temperature is usually based on the chemical structure of the PIL skeleton. Aromatic PILs generally have a higher initial decomposition temperature than aliphatic PILs. However, when conjugates are introduced into macromolecular chains containing five-membered pyrrolidines, the thermal stability is reduced because the double bonds are easily oxidized in the presence of oxygen. Due to the conjugated structure and steric hindrance, imidazole-based PIL has strong thermal stability, and its initial decomposition temperature value is higher than that of pyrrolidine salt PIL. The thermal stability of ammonium-based polycations is lower than that of imidazole and pyrrolidine PILs, but higher than that of phosphorus and sulfide salts. The thermal stability of PIL increases as the length of the substituents in the cation decreases, while extending the length of the alkyl chain between the polymer backbone and the ionic substituent results in a decrease in thermal stability.
The chemical structure of the anion also affects the initial decomposition temperature of PIL. By comparing poly(1-vinyl-3-ethylimidazole) PIL with different anions, it was found that the thermal stability decreases in the following order: CF3S > (CF3SO2) 2N->C12H25C6H4S >P >Br->C16H34P. This trend has also been found in other similar PILs and similar IL series.
PILs have certain resistance to chemical and electrochemical corrosion. However, in specific devices or under extreme reaction conditions, some side reactions such as structural rearrangements or degradation may also occur, and these effects must be studied in practical applications. For example, hydroxide ions in PILs may cause chemical instability due to their high nucleophilicity. For ammonium and phosphonium cations, C2- or N3-substituents can affect the chemical stability of imidazole cations under strongly alkaline conditions.
The electrochemical stability window of ILs is 2.5~5.0V. Similar to ILs, the electrochemical stability windows of PILs vary widely depending on the specific PIL cations and anions. The electrochemical stability of PILs differs from that of structurally similar ILs and in some cases even exceeds them. Studies have shown that the electrochemical stability of pyrrolidine-based PIL is better than that of imidazole-based PIL. Pyrrole cations have higher cathodic decomposition potentials than unsaturated cyclic quaternary ammonium cations.
Depending on the different structures and properties of PIL, it can be applied in the construction of polymer electrolytes, electrochemical devices, smart materials, catalyst supports, porous polymers, and antibacterial materials. Generally speaking, the synthesis methods of polyionic liquids are as follows.
(1) Free radical polymerization
Conventional free radical polymerization is the key and commonly used polymerization method to obtain PIL and composite materials. This method for high-volume polymerization is suitable for introducing a variety of functional groups and exhibits high tolerance to reaction mixture impurities. Standard polymerization methods have been widely used to prepare advanced materials based on IL and PIL.
(2) Living radical polymerization
In order to optimize the performance of PIL polymer materials, it has been possible to prepare PIL chains with predetermined molecular weights, small molecular weight distributions and ordered morphological structures. Because of this controllable free radical polymerization technology, target PIL can be “customized”.
Recently, various controlled polymerization techniques have been put into practice, such as addition-fragmentation chain transfer polymerization (RAFT), nitroxide radical polymerization (NMP), cobalt-mediated radical polymerization (CMRP), and organotelluride-mediated reactive Free radical polymerization (TERP), etc. Most of the PILs synthesized by these methods have a uniform degree of polymerization and a fairly narrow molecular weight distribution.
(3) Cationic polymerization
In the 1980s, the East Village and Kennedy groups discovered controllable living cationic polymerization. Controlled living cationic polymerization is usually carried out in organic solvents with initiators and co-initiators. Highly polar organic solvents enhance cationic polymerization due to the balance between active and dormant species. However, polar solvents, especially chlorinated solvents, are toxic, volatile and corrosive, which will cause a certain degree of environmental pollution. In addition, Lewis acid catalysts are difficult to separate from the reaction products.
Cationic polymerization of isobutyl vinyl ether (IBVE) in 1-octyl-3-methylimidazolium tetrafluoroborate ([OMIM][BF4]) was carried out at 0°C. Compared with polymerization in normal organic solvents, the cationic polymerization of IBVE in [OMIM][BF4] proceeds in a milder exothermic manner, resulting in polymers with higher molecular weights and yielding higher monomer conversions. Introducing a small amount of 2,6-di-tert-butylpyridine (DTBP) into the reaction system can make the polymerization reaction more controlled. In addition to the aggregation of ILs, it is also possible to immobilize ILs on substrates. Obviously, doing so will partially reduce the ion mobility and thus the ionic conductivity, but it will avoid the problem of liquid leakage while still having ion mobility. Figure 8-4 illustrates the process of cross-linking with IL within the polymer matrix. The results showed that although the ionic conductivity was reduced, the electrochemical stability was improved.
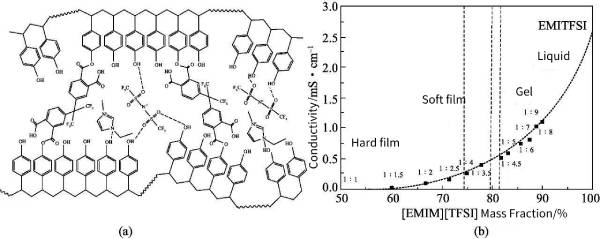
Figure 8-4 Polytetravinylphenol (C-P4Ph) and 1-ethyl-3-methylimidazole bistrifluoromethanesulfonimide
([EMIM][TFSI]) cross-linked structure (a) and the relationship between ionic conductivity and IL/polymer ratio (b)
Compared with ordinary nonionic polymers, PIL-based polymer electrolytes have higher ionic conductivity (up to 10-3S·cm-1 at 25°C), wider electrochemical windows (up to 5V), higher Thermal stability (up to 350°C), non-flammability and good compatibility. Therefore, PIL is widely used in various electrochemical devices, such as electrolytes for fuel cells, dye-sensitized solar cells, supercapacitors, electrochromic devices, transistors, etc.
Most single-cation electrolytes for Li metal batteries perform well only at 60 °C or above. In order to solve this problem, Zhang et al. prepared bipolar electrolyte PIL/LiTFSI. After using this electrolyte, lithium batteries can be operated at room temperature, which also enhances the safety of lithium batteries.
Lightweight supercapacitors are expected to use solid or quasi-solid polymer electrolytes instead of liquid electrolytes, thereby reducing sealing and housing requirements. Therefore, PIL electrolytes are considered as ideal solid-state electrolytes for supercapacitors. Solid polymer electrolytes generally have the following applications.
(1) Applied to all solid-state solar cells
In the development of all-solid-state solar cells, conductive polymers have great potential for development. Conductive polymers used in dye-sensitized solar cells (DSSC) solid electrolytes must meet the following conditions: the polymerization reaction occurs at a temperature where the dye does not desorb; the polymerization reaction can occur in the presence of iodine; the polymerization reaction does not require Initiator, to prevent the decomposition products of the initiator from degrading the performance of the battery.
Nogueira et al. used a copolymer of chlorohydrin/ethylene glycol with NaI and I2 to prepare an electrolyte and assembled a DSSC for research. Under illumination conditions of 0.1 sun, the photoelectric conversion efficiency is 2.6%.
The researchers added glutaraldehyde to the acetonitrile solution of polyethylene glycol, and added KI and I2 to cross-link to form a solid electrolytes, thus improving the contact with the TiO2 film. The ion conductivity of the cross-linked polymer was between 10-5 and 10 -3S·cm-1, the resulting battery achieved a photoelectric conversion efficiency of 3.64% under AM1.5 light intensity.
The volatilization of residual solvent in the polymer will form pores in the polymer, resulting in reduced ionic conductivity and poor contact between the electrolyte and the electrode. In order to overcome these difficulties, Nogueira et al. used a copolymer of epichlorohydrin and ethylene oxide, added NaI and I2 to prepare the electrolyte, heated the electrode to volatilize the residual solvent, and added the plasticizer polyethylene glycol methyl ether to fill the solvent after volatilization. The pores left. After heating, the stability of DSSC is greatly improved. The ion diffusion coefficient of electrolyte with plasticizer added is about 5 times higher than that without plasticizer. The photoelectric conversion efficiency of the obtained DSSC is also significantly improved.
Meng Qingbo et al. prepared a new type of solid electrolytes LiI(HPN)x (2≤x≤4) using lithium iodide and 3-hydroxypropionitrile, and carried out the application research of its DSSC. Under the light intensity of AM1.5, the battery The photoelectric conversion efficiency is 5.4%.
At present, the photoelectric conversion rate of DSSC prepared with conductive polymers is low, and it is necessary to further improve the interface contact, reduce the recombination of photogenerated charges, and improve the ion conductivity.
(2) Applied to supercapacitors
Dissolve poly(diallyldimethylammonium) bis-(trifluoromethanesulfonyl)imide in IL and use N-butyl-N-methylpyrrolidine bis(trifluoromethanesulfonyl)imide. Amines ([PYR14][TFSI]) are used as electrolytes in supercapacitors. Before assembling a supercapacitor, wetting the electrodes with electrolyte is a key process to improve carbon-electrolyte contact. However, these supercapacitors also suffer from poor performance due to poor electrode/electrolyte interface properties. The specific capacitance of supercapacitors can be further improved by using pyridine-based polyelectrolytes with high ionic conductivity.
Using PAN, PEO, and PVA as substrates, Lewandowski et al. used ionic liquids [EMIM][BF4], [BMIM][PF6], [BP][TFSI][N-methyl-N-propylpyridine-bis(trifluoro Methylsulfonyl)imide] was used as an ion source and a plasticizer to directly mix a series of ion-conducting polymer electrolytes, and then a series of electric double layer capacitors were assembled and tested. The maximum room temperature conductivity of this type of polymer electrolyte Up to 15×10-3S·cm-1, and the electrochemical window is about 3V (on glassy carbon electrode). The specific capacitance per unit area of activated carbon electrode is 4.2~7.7μF·cm-2, and the specific capacitance per unit mass is up to 200F·g-1.
8.3 Gel electrolyte
Gel is a colloidal dispersion system with a certain geometric shape and certain properties of solid and liquid. In gel electrolytes, the polymer is not only a supporting matrix but also an active colloidal component. Generally, the gel has a spatial network structure, which not only has strength, elasticity and yield value, but also allows ions to diffuse freely in it, and is semi-solid and has no fluidity. From the definition of gel, we can know that gel has the characteristics of both solid and liquid. Highly swollen gels have as large diffusion coefficients as liquids. The gel system will have the functions of adsorption, desorption, loading, separation, and sustained release of substances as the external environment changes, which neither solid nor liquid substances have.
Since the room temperature ionic conductivity of all-solid polymer electrolytes is very low and far from meeting the requirements for practical applications, in 1975, Feuillade and Perche proposed that the polymer be immersed in an organic solvent containing an alkali metal salt to form a condensate. Colloidal state, this gel state polymer electrolyte not only has the high ionic conductivity of liquid electrolytes, but also has good processing properties. The gel polymer electrolyte contains a large amount of liquid. The electrolyte salt is mainly dispersed in the liquid phase, and its ion transport also mainly occurs in the liquid phase. Its transport mechanism is similar to that of liquid electrolytes.
There are many types of polymer materials. As the skeleton material of gel polymer electrolyte, it must meet the following requirements: good film-forming properties, high film strength, wide electrochemical window, and good stability in organic electrolytes. Gel polymer electrolytes can be divided into two types, one is physically cross-linked gel polymer electrolyte, and the other is chemically cross-linked gel polymer electrolyte. The physical cross-linking type generally involves the self-assembly of linear polymer molecules, solvents, and salts through non-covalent bonding into a network structure, which in turn causes the entire system to gel and form a gel film. The chemically cross-linked type is a gel in the true sense. It first adds monomers and initiators to the organic electrolyte, and then polymerizes the monomers through heating or light radiation, forming a network structure through covalent bonds. This network structure constitutes a gel polymer electrolyte.
Gel polymer electrolyte is essentially a plasticizing system, which is a polymer expansion system formed by fixing solvent molecules between polymer chains. Gel polymer electrolytes are mainly composed of polymers, plasticizers and other parts, and function as both separators and ion carriers in batteries.
Solid electrolytes are another key component of flexible solid-state supercapacitors. Compared with liquid electrolytes, solid electrolytes are easier to handle, have higher reliability, and have a wider operating temperature range. In addition, the use of solid electrolytes avoids leakage issues, thus reducing the cost of device packaging. The most widely used solid electrolytes in supercapacitors are gel polymers. A good solid-state electrolyte should have the following advantages: low cost, nontoxicity, high ionic conductivity at ambient temperature, good mechanical strength, good stability, and a wide potential window. Compared with dry solid polymer electrolyte conductivity (10-8~10-7S·cm-1), gel polymer electrolyte exhibits higher ionic conductivity (10-4~10-3S·cm-1) .
Gel polymer electrolytes usually consist of a polymer skeleton as the main body, an organic/aqueous solvent as a plasticizer, and a supporting electrolyte salt. Polyacrylate (PAA), polyethylene oxide (PEO), polyvinyl alcohol (PVA), polyacrylonitrile (PAN), polyvinylidene fluoride (PVDF), polyvinylidene fluoride-co-hexafluoropropylene (PVDF-co-HFP) and polymethyl methacrylate (PMMA), poly(ethylene glycol) blended poly(acrylonitrile) (PAN-b-PEG-b-PAN) are used to prepare gel electrolytes Host polymer. Organic solvents commonly used as plasticizers are ethylene carbonate (EC), propylene carbonate (PC), ethyl methyl carbonate (EMC), dimethyl carbonate (DMC), diethyl carbonate (DEC), Gamma-butyrolactone (GBL) and tetrahydrofuran (THF). Usually, two or more organic solvents are mixed to form plasticizers to obtain higher ionic conductivity, lower viscosity and wide stable potential window. In gel polymer electrolytes, the electrolytic salt should have large anions and low dissociation energy to provide free/mobile ions. Gel polymer electrolytes can be divided into three types: ① lithium ion gel polymer electrolytes; ② proton-conducting gel polymer electrolytes; ③ other ion gel polymer electrolytes.
(1) Lithium-ion gel polymer electrolyte Lithium-ion gel polymer electrolyte is commonly used in lithium-ion batteries and supercapacitors. To date, various Li-ion gel polymer electrolytes include PMMA/LiClO4, PAN-b-PEG-b-PAN/LiClO4, PVA/LiClO4, PVA/LiCl, P(VDF-co-HFP)/SiO2(Li+) , PAE/PVA/SiO2/LiClO4 are used in solid-state supercapacitors. Lithium-ion gel polymer electrolytes are usually prepared by mixing polymers (PMMA, PVA, PAN, etc.) with lithium salts dissolved in organic solvents at temperatures ranging from 70 to 170°C. Huang et al. recently designed a gel polymer electrolyte with high ionic conductivity and large working potential, using polyacrylonitrile (PAN-b-pegb-PAN) as the backbone and dimethylformamide (DMF) as the Plasticizer, LiClO4 serves as an electrolytic salt [Figure 8-5(a)]. The ionic conductivity of this gel electrolyte is very high, reaching 6.9×10-3S·cm-1, and the electrochemical window can reach 2.1V [Figure 8-5(b)].
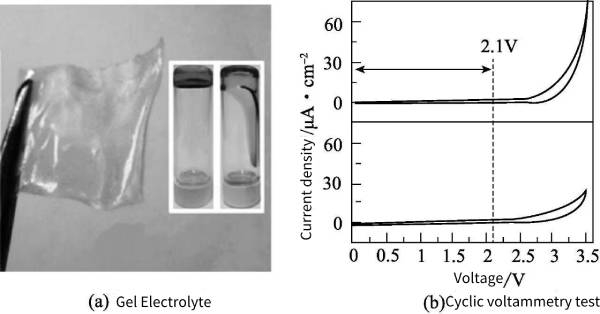
Figure 8-5 LiClO4 gel electrolyte
Organic solvents are often toxic, flammable, and expensive. Therefore, it is necessary to replace organic solvents with hydrogel polymer electrolytes. Using hydrogel electrolytes can also significantly reduce device costs. Recently, Wang et al. designed an aqueous gel polymer electrolyte using PVA as the main body, LiCl as the electrolytic salt, and distilled water as the solvent. This aqueous and neutral LiCl/PVA gel electrolyte can effectively inhibit the chemical dissolution of the electrode and the irreversible electrochemical oxidation reaction. Using this LiCl/PVA gel as an electrolyte can improve the cycling stability of VOx and VN electrodes. For example, the VOx electrode maintains excellent capacitance after 5000 cycles without losing the electrochemical performance of VOx.
(2) Proton-conducting gel polymer electrolyte Proton has a higher mobility than Li+, which makes it more promising as a charge carrier for fast charging/discharging supercapacitors. Proton-conducting polymer electrolytes are typically prepared by dipping a polymer matrix in a solution with a polar solvent. H2SO4, H3PO4, and heteropolyacids are commonly used as proton donors in proton-conducting polymer gel electrolytes. At present, a large number of proton-conducting polymer gel electrolytes have been developed, such as PVA/H2SO4, PVA/H3PO4, PVA/H3PO4/cellulose silicotungstate/chitin/1-propenyl-3-methylimidazolium bromide /H2SO4. Their room temperature ionic conductivities range from 10-4 to 10-2S·cm-1. Lian et al synthesized silicotungstic acid (SiWA)/PVA/H3PO4 gel electrolyte with excellent performance and long life. Compared with Nafion, SiWA/PVA/H3PO4 gel electrolyte shows higher conductivity, reaching 8mS·cm-1 under suitable environmental conditions. Wu et al. prepared redox-mediated gel polymer electrolytes by adding hydroquinone (PB) to PVA-H2SO4 gel polymer electrolytes. By adjusting the amount of hydroquinone, the ionic conductivity of PVA/H2SO4/PB can reach 34.8mS·cm-1. In addition, a new proton-conducting polymer gel electrolyte has recently been designed that contains silicotungstic acid (SiWA) in cross-linked polyvinyl alcohol. Flexible solid-state supercapacitors using this polymer gel electrolyte have extremely high rate capacity (50V·s-1).
(3) Alkaline gel polymer electrolytes In recent years, alkaline gel polymer electrolytes have attracted increasing attention due to their great potential in all-solid-state alkaline rechargeable batteries and supercapacitors. Scientists have made exciting progress in the preparation of versatile alkaline gel polymer electrolytes, including poly(epichlorohydrin-co-ethylene oxide)/KOH/H2O, PEO/KOH/H2O, Potassium poly(acrylic acid)/KOH/H2O and PVA/KOH/H2O. The researchers studied the PEO/KOH polymer electrolyte system through impedance and cyclic voltammetry. The research results show that the best conductivity (10-4~10-3S·cm-1) can be achieved by controlling the ratio of the three components of PEO/KOH/H2O polymer electrolyte. Yang et al. reported the synthesis of PVA-based gel polymer electrolytes doped with KOH, and the resulting alkaline PVA/KOH/H2O polymer electrolyte had a conductivity of 10-2S·cm-1.
(4) Other ionic gel polymer electrolytes In addition to the aforementioned gel polymer electrolytes, several other types of composite electrolytes have also been reported. For example, Matsuda et al. prepared polyacrylonitrile, propylene carbonate and tetraalkylammonium salts (tetrabutylammonium perchlorate, tetraethylammonium perchlorate and tetraethylammonium tetrafluoroborate). Gel polymer electrolyte. In addition, Lee et al. also reported polyacrylate (PAAK) (PAAK:KCl:H2O=9.0%:6.7%:84.3%) electrolyte with a conductivity of 10-1S·cm-1.
Ionic liquid gel is a new type of polymer functional material prepared by introducing ionic liquid into polymer materials. From the most basic point of view, ionic liquid gel materials consist of two components: an ionic liquid and a continuous solid phase. Compared with ordinary hydrogels, in addition to the network structure and environmental responsiveness of hydrogels, ionic liquid gels also have the good stability and strong conductivity of ionic liquids themselves, which can maintain their original characteristics. . Ionic liquids can act both structurally and functionally, giving gel materials new life. At the same time, the ionic liquid gel material also provides a symbiotic relationship between the dispersed ionic liquid phase and the solid continuous phase.
Ionic liquids have many advantages over traditional electrolytes. For example: ① low vapor pressure, almost non-volatile; ② liquid in a wide temperature range, good thermal stability, non-toxic; ③ through the selection of anions and cations, it can control its reaction to inorganic substances, water, organic substances and polymerization. ④It has good electrical conductivity and wide electrochemical window, and can be used as an electrolyte for many substances in electrochemical research; ⑤The acidity and polarity can be adjusted. At present, ionic liquids have been widely used in chemical synthesis and electrochemistry.
There are many ways to synthesize ionic liquid gels. Among them, the gel can be prepared by in situ polymerization of ethylene monomer, and the flexible film can be formed by dissolution and casting. Gels can also be formed from natural polymers (biological macromolecules). Kadokowa et al. synthesized a gel composed of ionic liquid (1-allyl-3-methyl-3-methylimidazole amide) and chitin/cellulose. Kang et al. also designed an ionic liquid gel electrolyte by mixing silica nanopowder and ionic liquid 1-ethyl-3-methylimidazole bis(trifluoromethylsulfonyl)imide through pyrolysis. Recently, Chen et al. incorporated 1% (mass fraction) graphene oxide in P(VDF-HFP)-1-ethyl-3-methylimidazolium tetrafluoroborate (EMiMbF4) ionogel. Kang et al. also synthesized a flexible supercapacitor based on ionic liquid polymer gel electrolyte and carbon nanotube electrode materials. Figure 8-6 shows the ionic gel.
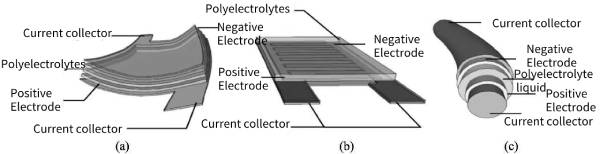
Figure 8-6 Ion gel
In the initial ionic liquid gel prepared by the sol-gel method, the ionic liquid is used as a template, and the ionic liquid is removed after the material is formed. In similar ionic liquids, which have been successfully used as templates to prepare mesoporous solids, the IL is removed after the material is ready. The complete porous structure is revealed when the IL is removed. The properties and quality of the ionic liquid contained in the silica ion gel have a direct impact on the structure of the solid continuous phase. Inorganic oligodimethylsiloxane (PDMS) was synthesized based on triethoxysilane end groups. This sol-gel precursor was then used to form a gel with 80% [EMIM][FSI]. The cations of IL are combined with silane oxygen to form a mixed sol-gel precursor, the (anion-associated) sol-gel precursor is mixed with tetramethoxysilane (TMOS), and the sol-gel reaction starts. In this case, no free ionic liquid is present as the dispersed phase, and the only liquid present is as a by-product of the sol-gel reaction (ethanol and methanol). The conductivity of the thin film electrolyte made of 70% IL and 30% epoxy resin is about 1mS·cm-1. On the other hand, the tunable hydrophobicity/hydrophilicity of ILs facilitates the synthesis of more stable gel polymers. Since Fuller et al. began to explore ILs-based gel polymer electrolytes, many researchers have applied them in various electrochemical systems, especially in lithium-ion batteries and supercapacitors, and it has become the choice for designing flexible solid-state supercapacitors. one. The polymer matrix doped ILs can improve its thermal stability and increase the electrochemical window to 3.5-4.0V. Many polymer gel electrolytes have been used in supercapacitors, such as polyacrylonitrile, polyethylene hexafluoropropylene, polyvinylidene fluoride/polyvinylacetate, polyethylene oxide, polyvinyl alcohol, polymethyl Methyl acrylate and polytetrafluoroethylene, chitosan, etc.
Although gel polymers are in a solid state, their thermodynamic properties and mechanical stability are not ideal. The high-viscosity IL facilitates the gelation process and contributes to the mechanical integrity of the gel polymer, which can improve the bending ability of flexible supercapacitors and facilitate the fabrication of yarn supercapacitors. Many research groups have made continuous attempts to prepare a series of conductive polymer flexible battery materials with excellent properties. Figures 8-7 show gel electrolyte supercapacitors in flexible sandwich battery configuration (a), interdigitated battery configuration (b) and coaxial fiber optic battery configuration (c), respectively. Meng et al. of Tsinghua University prepared a carbon nanotube/polyaniline composite flexible electrode by conducting electropolymerization on a self-supporting carbon nanotube film. A solid-state supercapacitor assembled using this composite electrode and PVA/H2SO4 sol electrolyte is only as thick as A piece of A4 paper, in a highly bent state, the integrated device can reach a specific capacitance of 350F·g-1 based on polyaniline (PANI), with almost no attenuation after 1000 cycles. Based on the quality of the entire device, the supercapacitor can reach The specific capacitance of 31.4F·g-1 is about 6 times that of commercial high-current capacitor products.
Figure 8-7 Gel electrolyte supercapacitor
Kim from the University of Wollongong in Australia deposited a gold film on a PVDF flexible film by electrodeposition, and prepared a PPy/Au/PVDF flexible film. In a 0.2mol L-1 NaPTS electrolyte, the specific capacitance can reach 370F g- 1.
Nakagawa et al. mixed [EMIM] [BF4] and LiBF4 to prepare a binary room temperature molten salt (i.e., room temperature ionic liquid) LiEMIBF4, and then added cross-linked polyoxyethylene PEO to it to form a gel polymer electrolyte (GLi EMIBF4) , and assembled Li[Li1/3Ti5/3]O4/LiEMIBF4/LiCoO2 batteries and Li[Li1/3Ti5/3]O4/GLiEMIBF4/LiCoO2 batteries.
Lu et al. from Nanjing University of Aeronautics and Astronautics co-filtered polypyrrole@carbon nanotubes and graphene to prepare a GN-PPy/CNT flexible composite film with evenly dispersed active materials. At a current density of 0.2A·g-1, the mass ratio The capacitance is 211F·g-1, and the volume specific capacitance is 122F·cm-3.
Laforgue from the Functional Polymer Research Group of the National Research Council of Canada’s Institute of Industrial Materials prepared a self-supporting poly(3,4-ethylenedioxythiophene) film through electrospinning. The film itself has good electrical conductivity and electrochemical activity. The conductivity of the conductive film reaches 60S·cm-1, and the electrochemical activity is also improved compared with previous reports. Panzer et al. prepared UV-initiated cross-linked poly(ethylene glycol) diacrylate (PEGDA) ionic liquid gel.
Although flexible electrodes based on various conductive polymers have been prepared, these electrolytes have high requirements for electrodes, and some require the use of carbon nanotubes and gold and other precious metals, which are very expensive, and the preparation process of graphene is complicated. The instability of the flexibility of pure conductive polymer films also greatly limits their development and application. Therefore, developing flexible polymer-based electrodes with excellent electrochemical performance while maintaining low cost is still a difficult problem that researchers need to overcome.
8.4 Composite solid polymer electrolyte
8.4.1 Adding inorganic material type solid polymer electrolyte
The aforementioned electrolytes have been hindered from practical applications due to various limitations. Therefore, there is a need to improve solid-state electrolytes in an efficient manner. The composite electrolyte is composed of conventional electrolyte and other solid substances. In 1982, Weston et al first introduced inorganic ceramic fillers into the polymer to synthesize the PEO-LiClO4 system, and modified the solid polymer electrolyte by introducing inorganic powder α-Al2O3. The experiment found that after adding 10% α-Al2O3, The mechanical properties of the polymer electrolyte are greatly improved. Since then, people have gradually begun to explore the role and influence of inorganic fillers on polymer electrolyte systems, and thus formed a new polymer electrolyte system called composite polymer electrolyte.
Not only that, the researchers also conducted further research on the conductive mechanism of the polymer electrolyte. Using DSC, NMR, AC impedance and other techniques, it was shown that ions migrate in the crystal phase, but the process of ion conduction mainly occurs in the amorphous phase. within the phase area. The polymer electrolyte realizes its conduction process through the movement of chain segments leading to the “complexation-dissociation-recomplexation” of ions. The ethylene oxide chain segment of PEO can dissolve lithium salt and complex with Li+ to dissolve lithium salt; above the glass transition temperature (Tg), the PEO molecular chain is flexible and the chain segment moves fast. Therefore, polyethylene oxide (PEO) is an ideal polymer matrix for an inorganic composite polymer electrolyte, and most studies are based on PEO.
Kumar et al. have conducted in-depth research on the influence of inorganic fillers on the ionic conductivity and electron transport mechanism of polymer electrolytes. Inorganic fillers used in the modification of polymer electrolytes can generally be divided into two categories: one is neutral inorganic fillers that do not have electrical conductivity, such as SiO2, Al2O3, MgO, ZrO2, TiO2, etc.; Inorganic fillers with ion transport capabilities, such as γ-LiAlO2, Li3N and other silicate inorganic fillers. These inorganic fillers can increase the degree of dissociation of electrolyte salts and increase the velocity of ion movement in polyelectrolytes; improve the mechanical properties and thermal properties of the polymer matrix; destroy the original regular arrangement of polymer chain segments, making it maintain an amorphous and adsorption system Medium and trace impurities reduce electrode polarization and corrosion.
Because BaTiO3 can promote the dissociation of lithium salts, weaken the interaction between lithium ions and oxygen, thereby increasing the conductivity. After adding 10% BaTiO3 into the PEO-Li TFSI system by Itoh et al., the conductivity can reach 2.6×10-4S cm-1 at 30°C and 5.2×10-3S cm-1 at 80°C. , and the stable temperature in air reaches 312°C. Capiglia found that the heat treatment temperature of SiO2 has a great influence on the conductivity of the polymer electrolyte. Adding 5% nano-SiO2 treated at 900°C to PEO-Li TFSI, the conductivity at room temperature is 1.4×10-4S cm-1. When using SiO2 treated at 100°C, the conductivity is only 4.6×10-5S under the same conditions · cm-1.
Weston et al. first proposed adding inert particles to the polymer electrolyte, the purpose of which was to improve the mechanical strength of the polymer electrolyte. However, during the research process, it was found that the addition of inert particles could significantly improve some other properties of the polymer electrolyte, which caused aroused great interest of researchers.
In addition, it can be improved by introducing lithium salts with high conductivity and excellent performance (such as LiAsF6, LiBF4, LiPF6, LiSCN, LiClO4) and polyethylene oxide (PEO) to destroy the crystallization of polymers and increase the proportion of amorphous forms. Conductivity.
Xi et al. synthesized composite polymer electrolytes using SBA mesoporous molecular sieves as filling materials. SBA-15 mesoporous molecular sieve has a two-dimensional hexagonal phase pore size, and the micropores of some mesoporous channels are connected to each other. Its structure is shown in Figure 8-8. The DSC study of this composite electrolyte found that SBA-15 can significantly reduce the crystallinity and glass transition temperature of PEO. The Lewis acid site on the SBA-15 skeleton structure can undergo a Lewis acid-base interaction with the ether O in the PEO segment and the O atom in Cl, thereby releasing more mobile ions and improving the conductivity.
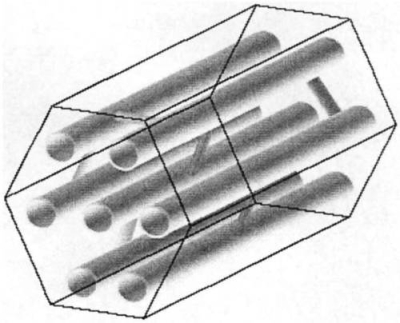
Figure 8-8 SBA-15 structure
The addition of inorganic fillers can effectively inhibit the crystallization behavior of polymer molecular chains, thereby increasing the proportion of polymer amorphous regions and reducing the crystallinity and glass transition temperature of polymers. At present, researchers focus on finding effective inorganic fillers, hoping to essentially explore the microscopic interactions between polymer-inorganic particles in composite polymer electrolytes, and strive to design composite materials with specific functions on this basis. polymer electrolyte.
8.4.2 Add plasticizer type composite polymer electrolyte
In order to improve the conductivity of the all-solid polymer electrolyte at room temperature, an organic electrolyte or a liquid plasticizer can be added to the polymer. When the plasticizer is added to the polymer, the original solid polymer electrolyte becomes a gel-state polymer electrolyte, and the conductivity can be increased by two orders of magnitude compared with the original, but the addition of a large amount of plasticizer will make The mechanical properties of polymer electrolytes are greatly reduced. 70%~80% polymer and 10%~12% plasticizer can form a polymer/plasticizer type with certain mechanical strength and room temperature conductivity between 10-4~10-3S·cm-1 composite polymer electrolyte. The function of the plasticizer is to create pores, which can reduce the crystallinity of the polymer, improve the mobility of the polymer segment, and reduce the activation energy of ion transmission. Commonly used plasticizers are ethylene carbonate (EC), propylene carbonate (PC), dibutyl phosphate (DBP), etc., and a mixture of several plasticizers can also be used. Compared with the polymer electrolyte without plasticizer, the room temperature ionic conductivity of the polymer/plasticizer composite polymer electrolyte formed by adding plasticizer will be improved. The polymer/plasticizer type composite polymer electrolyte system contains a large amount of organic plasticizer (such as PC, EC, etc.), which is easy to form a passivation film when the battery is working, which makes the charge/discharge capacity of the polymer lithium-ion battery and the battery life Life decay.
8.4.3 Polymeric composite polymer electrolyte
Polyethylene oxide (PEO) is easy to complex with alkali metal salts, and has excellent conductivity in polymers, so it is usually blended and modified for PEO and its derivatives when compounding. The blending can not only reduce the crystallinity of PEO, make a new breakthrough in the conductivity of the composite polymer electrolyte, but also enhance the mechanical properties of the composite polymer electrolyte. It is usually blended with polyvinylidene fluoride (PVDF), polypropylene oxide (PPO), polyacrylonitrile (PAN), polyethylene glycol (PEG), etc. However, the room temperature conductivity of this polymeric composite polymer electrolyte is still low, usually around 10-5S·cm-1, which is not enough to meet the practical requirements.