1 Types of aluminum-based solid solutions in electrolytic capacitor aluminum foil
Aluminum foil for electrolytic capacitors usually needs to be made of high-purity aluminum. However, even the highest purity aluminum inevitably contains a small amount of impurity elements. Sometimes it is necessary to artificially add a small amount of alloying elements to the aluminum foil for electrolytic capacitor aluminum foil to improve the various properties. The aluminum content in the aluminum foil for electrolytic capacitors is above 99.99% (mass fraction), so both impurity elements and alloying elements are trace elements, and their total amount will not exceed 0.01%. At this time, trace elements are often measured in parts per million.
Table 1 gives the composition analysis of some high-voltage anode aluminum foils produced at home and abroad. It can be seen that the most common impurity elements in the for electrolytic capacitor aluminum foil are Fe, Si, and Cu,but sometimes it is also necessary to pay attention to the content of some other elements such as Mg and TiNn, their state in the aluminum foil, and their possible impact on the quality of the aluminum foil for electrolytic capacitors. The electrochemical and dielectric properties of aluminum and its oxides are mainly used in the manufacture of aluminum electrolytic capacitors. Therefore, as a functional material, the chemical composition of aluminum foil will often have a great impact on the performance even if the content changes by only about 10-6.
Table 1 Composition analysis of some high-voltage anode aluminum foils produced at home and abroad
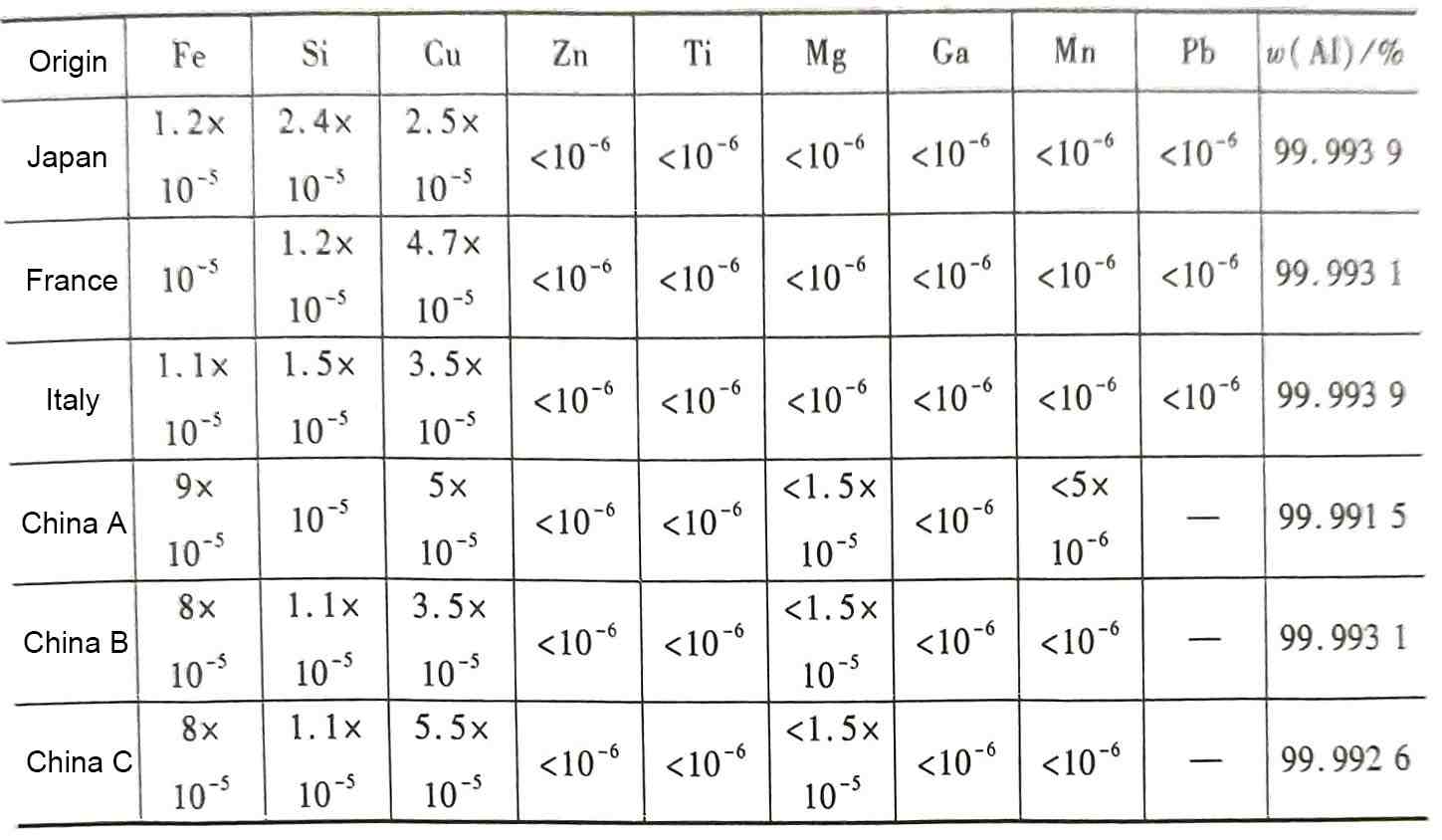
Figure 1 gives examples of four binary alloy phase diagrams of Al-Fe, Al-Si, Al-Cu, and Al-Ti. It can be found that a small amount of Fe, Si, Cu, and Ti elements can be dissolved in aluminum.
A certain amount or even a large amount of other elements can usually be dissolved in aluminum crystals, thus forming a solid solution, which is called a solid solution or a primary solid solution. In a multi-element system, a single crystal usually appears at the end of the equilibrium phase diagram (Figure 1), so it is also called an end solid solution. A compound crystal composed of multiple elements must appear in the middle part of the equilibrium phase diagram. If the solubility of the crystal for other elements is extremely limited, and the stoichiometric composition of this crystal can hardly change when it exists, it is called a stoichiometric compound; if the compound crystal allows a certain range of composition changes, it is called an intermediate phase, also known as a secondary solid solution. Within the composition range of the intermediate phase, an ideal compound composition can usually be determined as its identification component. The FeAl3 and TiAl3 in Figure 1 are intermediate phases, and the actual CuAl2 is also an intermediate phase. The matrix crystal phase in the solid solution is called the solvent of the solid solution, and the other elements dissolved in the matrix crystal structure are called solutes.

Figure 1 Aluminum-based binary phase diagram: (a) Al-Fe; (b) Al-Si; (c) Al-Cu; (d) Al-Ti
According to the position of the solute atoms in the solvent crystal structure, the solid solution can be divided into substitutional solid solution and interstitial solid solution, also known as substitutional solid solution and interstitial solid solution. In substitutional solid solution, the solute atoms replace the position of the solvent atoms in the crystal structure [Figure 2 (a)]; in interstitial solid solution, the small-sized solute atoms are in the interstitial position of the solvent crystal structure [Figure 2 (b)]. The radii of hydrogen, oxygen, nitrogen, carbon, boron and other atoms are 0.046 nm, 0.060 nm, 0.071 nm, 0.077 nm and 0.097 nm respectively, all less than 0.1 nm, and belong to small-sized elements. When the atoms of these elements are dissolved into the solid solution, they are usually located in the interstitial position of the solvent crystal structure, thus forming an interstitial solid solution. Most of the solute elements dissolved into the matrix in of the electrolytic capacitor aluminum foil replace the position of aluminum atoms, so what is formed is a substitutional solid solution.

Figure 2 Solute atoms in the solid solution (gray atoms): (a) substitutional solid solution; (b) interstitial solid solution
The solute atoms in the substitutional solid solution usually replace the solvent atoms in different positions in a disordered and random manner. The substitutional solvent atoms can be approximately understood as a small number of point defects in an ideal crystal. This point defect will increase the critical shear stress τc required for the dislocation to start slipping, thereby increasing the strength of aluminum. Many factors such as the size difference, electronegativity difference, and valence electron concentration of the solute and solvent atoms will affect the solubility of the solid solution. There is always a difference in the size of solute and solvent atoms. When solute atoms replace solvent atoms in the crystal structure, the lattice is distorted, elastic strain is generated around the solute atoms, and the unit cell constant of the solid solution is changed. The lattice distortion caused by the solute atoms has an important influence on the mechanical properties of the solid solution. If δ is used to represent the relative difference between the radius of the solute atom (rquality) and the radius of the solvent atom (rquality), then δ = (rquality-rquality) / rAgent. It is generally believed that when δ exceeds 15%, the solid solubility that can be achieved by the solid solution will be very limited. The greater the electronegativity difference between the solute atoms and the solvent atoms, the easier it is to form a stable compound, thereby reducing the solid solubility of the solute. A larger solid solubility can be obtained when the relative electronegativity difference is less than 0.5.
Figure 1 shows that the possible solubility of Fe, Si, Cu, and Ti in the face-centered cubic aluminum matrix is not high. At 655℃, Fe can be dissolved in the aluminum matrix at 0.04%, Si can be dissolved in the aluminum matrix at 577℃ at 1.59%, Cu can be dissolved in the aluminum matrix at 548℃ at 5.7%, and Ti can be dissolved in the aluminum matrix at 1.3% at 665℃. However, it is still difficult to accurately determine the solubility of each element of Fe, Si, Cu, and Ti under low temperature or room temperature conditions for electrolytic capacitor aluminum foil processing or service; because at low temperature conditions, there will be segregation, making the solid solution state of foreign atoms very complicated.
Analysis shows that the solid solubility of Fe at 427℃ is less than 0.01%, the solid solubility of Si at 227°C also reaches 0.01%, the solid solubility of Cu is 0.18% at 227°C and 0.05% at 22°C, and the solid solubility of Ti at 527°C is 0.2%~0.3% (Figure 1). It can be seen that Fe can hardly be dissolved in the aluminum matrix at room temperature, the room temperature solubility of Si and Ti is also very low, and Cu can be dissolved in a small amount at room temperature. When the content of these elements increases, second phases such as Si, FeAl3, CuAl2, and TiAl3 may appear in the alloy. The second phase is often distributed in the matrix of aluminum crystals in the form of dispersed particles, and its electrode potential and chemical properties are very different from those of the aluminum crystal matrix. During the corrosion process, the second phase particles in the matrix may cause the corrosion process to be more concentrated in its vicinity, destroying the uniformity of corrosion pores, and even damaging the mechanical properties of the electrolytic capacitor aluminum foil. Therefore, electrolytic capacitor aluminum foil is usually designed to be high-purity aluminum to prevent the appearance of the second phase and ensure the long-term stability and reliability of the capacitor.
Compared with other binary phase diagrams, it can be seen that among the other elements listed in Table 1, Mg and Ga have a large equilibrium solid solubility in the aluminum matrix; the solubility of Mn is similar to that of Si, which is relatively low; the solubility of Pb is similar to that of Fe, and it can hardly be dissolved. Zn has a special solid solution behavior. It can dissolve more than 60% in Al at high temperature, but it can hardly dissolve in equilibrium at low temperature.
In addition, the presence of elements such as Fe, Si, and Cu will slightly reduce the melting point of pure aluminum, while some elements such as Ti and Cr will slightly increase the melting point of pure aluminum (Figure 1).
2 Equilibrium segregation of solid solution elements
Whether it is a substitutional solid solution or an interstitial solid solution, the dissolution of solute atoms will cause volume changes in the microscopic area and the corresponding normal stress field: this stress field will interact with the normal stress field of the edge dislocation. There is no normal stress component in the stress field of the screw dislocation, so there will be no interaction due to the volume change caused by the solute atoms. If the volume change caused by the solute atoms is ΔV, the stress field of the dislocation has a hydrostatic pressure p=(бxx+бyy+бzz)/3. Under the first-order approximation condition, the interaction energy between the dislocation and the solute atoms is E=pΔV. For the interstitial solute atoms existing in the gaps of the solvent, ΔV can be regarded as the change caused by the presence of an interstitial solute atom in a crystal. For the substitutional solute atoms, let the atomic radius of the solvent be R and the atomic radius of the solute be R’, then the size mismatch ε=(R’-R)/R; the volume change caused by one solute atom ΔV=4πR3ε. Then the hydrostatic pressure p is
![]() (1)
(1)
The interaction energy between the edge dislocation and the solute atom is
![]() (2)
(2)
It can be seen that when the solute atom is larger than the solvent atom, that is, ε>0, E<0 in the lower half of the edge dislocation (π<θ<2π), that is, the solute atom is attracted to the lower side of the edge dislocation; on the contrary, if the solute atom is smaller than the solvent atom, it is attracted to the upper side of the extra half atomic plane. The solute atoms will move to the dislocation with the help of a certain migration process and gather around it, which is called the Coriel gas cluster; the emergence of this phenomenon is due to the elastic interaction between the dislocation and the solute atoms. For interstitial solute atoms, their size is generally larger than that of solvent gaps, so they are enriched on the lower side of dislocations. Figure 3 shows a schematic diagram of solute atoms forming Cottier gas clusters near dislocations. The formation of Cottier gas clusters and the gathering and segregation of atoms near edge dislocations are accompanied by a decrease in dislocation energy, so this segregation is a thermodynamic equilibrium segregation.
Most of the solute elements dissolved into the matrix in the of electrolytic capacitor aluminum foil are substitutional solid solutions, so their Cottier gas clusters are mostly in the form shown in Figure 3 (a). For example, the unit cell constant of Cu with the same face-centered cubic structure is a=0.361 53 nm, which is smaller than the unit cell constant of Al. It is generally believed that its atomic radius should be smaller than the radius of aluminum atoms. Therefore, when Cu atoms form Cottier gas clusters, they tend to be on the side of the extra half-atomic plane of the edge dislocation [see the dislocation in the lower right corner of Figure 3 (a)], forming a confrontation with the tensile stress zone below the dislocation. The standard electrode potential of thermodynamically stable Cu atoms is higher than that of thermodynamically unstable A1 atoms. Therefore, the combination of Cu atoms and the tensile stress zone of dislocations may further promote corrosion pore formation near dislocations.

Figure 3 Schematic diagram of the formation of Cottier gas clusters due to the aggregation of solute atoms near dislocations:(a) Substitution atoms; (b) Interstitial atoms
When a dislocation with Cottier gas clusters slides, the equilibrium position of the gas clusters relative to the dislocations will change, thereby increasing the elastic strain energy of the system. Therefore, the gas clusters have a dragging resistance to the dislocation sliding and play a pinning role on the dislocations. If the dislocation slides very slowly, the gas clusters can fully follow the migration, and the resistance of the gas clusters to the movement of the dislocations is very small; if the dislocation moves very quickly, the gas clusters cannot keep up with the dislocations, that is, the dislocations get rid of the gas clusters, and the resistance of the gas clusters to the movement of the dislocations is also very small; only when the dislocations move at a moderate speed, the dislocations are forced to drag the gas clusters with them, and the resistance of the gas clusters to the movement of the dislocations is the greatest at this time.
Figure 4 (a) shows the relationship between the grain boundary energy γ and the misorientation angle θ measured for some symmetrical tilted grain boundaries of aluminum, and Figure 4 (b) shows the relationship between the grain boundary energy γ and the misorientation angle θ calculated theoretically, and the two are basically consistent. When the misorientation angle changes to certain specific values, more special atoms will appear on the tilted grain boundary, and their positions also conform to the rules of atomic arrangement in the grains on both sides of the grain boundary. This phenomenon reduces the energy of the grain boundary at these large misorientation angles, as shown in Figure 4 (b), which shows several grain boundary energy reduction points in the large-angle grain boundary range in the middle of the calculation curve. However, this phenomenon of grain boundary energy reduction has not been measured in actual aluminum materials [Figure 4 (a)].
The grain boundary structure is looser than the intracrystalline structure, and the energy of solute atoms in the crystal is higher than that at the grain boundary, so the solute atoms tend to spontaneously segregate to the grain boundary and cause grain boundary segregation. The segregation of impurity elements in aluminum at grain boundaries will significantly reduce the grain boundary energy; however, at the above-mentioned special misorientation grain boundaries, the strong regularity of atomic arrangement reduces the amount of impurity segregation and the magnitude of grain boundary energy reduction. The different segregation behaviors of impurity elements at these two grain boundaries make the grain boundary energy in the large misorientation range tend to be consistent, and do not show obvious differences [Figure 4 (a)]. The grain boundary segregation of impurity elements will cause the grain boundary energy to decrease, so grain boundary segregation is also a thermodynamic equilibrium segregation. As the temperature increases, the distribution difference caused by the energy difference of solute atoms in the crystal and at the grain boundary weakens, so the grain boundary segregation also weakens. This has an important impact on the quality and performance for electrolytic capacitor aluminum foil.
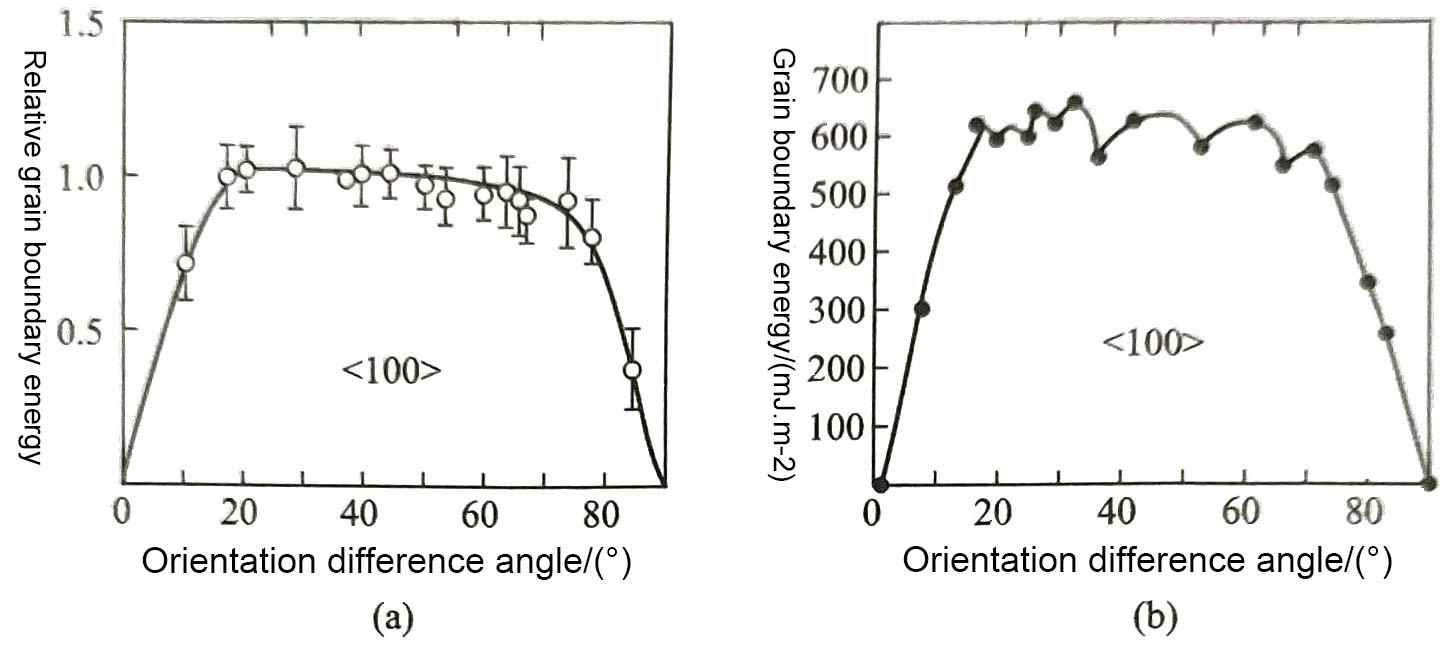
Figure 4 Grain boundary energy of a symmetrical tilted grain boundary with <100> as the rotation axis in aluminum at 650℃
(a) Measured value and (b) calculated value
Surface segregation refers to the enrichment of solute atoms in the solid phase from the condensed phase to the solid/gas phase interface. The free energy of solute atoms on the surface is lower than that in the crystal, and the free energy difference is the driving force for solute atoms to move toward the surface. This segregation is a thermodynamic equilibrium segregation, and the concentration of segregated solute atoms is highest on the surface, and the concentration decreases rapidly from the surface to the crystal.
On the other hand, adsorption is an important physical phenomenon on the metal surface. Surface adsorption refers to the accumulation of atoms from the gas phase or liquid phase to the solid/gas phase or solid/liquid phase interface in a two-phase system containing a solid phase and a liquid phase or a gas phase. The adsorbed atoms can also evaporate again from the surface, which is called desorption. A stable surface is in a state of equilibrium between adsorption and desorption. Adsorption changes the composition and structure of the surface and interface, and these changes will cause a series of physical, chemical and mechanical properties of the aluminum surface. Surface adsorption can be divided into physical adsorption and chemical adsorption. When adsorption occurs, it is an energy reduction process, so it is also a process tending to thermodynamic equilibrium. It is especially important for electrolytic capacitor aluminum foil, because its surface characteristics directly affect the performance of electrolytic capacitors.
Every atom is polarizable to some extent. When it approaches the surface, the surface atoms and the adsorbed atoms polarize each other, which reduces the energy of the system. Mutual polarization leads to small dipole attraction, forming van der Waals force. Physical adsorption is the adsorption of adsorbed atoms on the solid/gas interface by van der Waals force. Since the van der Waals force is weak, the structure of the surface and the adsorbate does not change much after physical adsorption. The heat of physical adsorption is low, at several thousand joules per mole, so physical adsorption is the main surface at low temperatures.
Chemical adsorption is similar to a chemical reaction. Electron transfer occurs between the atoms of the adsorbed crystal and the adsorbate, changing the surface structure. During chemical adsorption, the force between the adsorbed crystal and the molecules or atoms of the adsorbate is mainly electrostatic Coulomb force. In chemical adsorption, if complete electron transfer occurs between the adsorbed crystal and the adsorbate, so that the atoms or molecules of the adsorbed crystal and the adsorbate become ions, then the bond between the two is a pure ionic bond, which is called ionic adsorption. If the electron transfer between the adsorbed crystal and the adsorbate is incomplete in chemical adsorption, and one or both of them provide electrons as shared electrons, local valence bonds such as covalent bonds, ionic bonds or coordination bonds will be formed. At this time, the shared electrons between the two are not equivalent, which is called chemical bond adsorption. The binding force of chemical adsorption is mainly the Coulomb force between shared electrons and ions, so the adsorption heat is much higher than that of physical adsorption, which is tens of kilojoules per mole. In actual chemical adsorption, in addition to the above two situations, there are also situations where both occur. These adsorption phenomena have an important influence on the surface characteristics of electrolytic capacitor aluminum foil, affecting its performance and reliability.