1 Characteristics of electrolytic aluminum liquid
Electrolytic aluminum liquid is produced by high-temperature electrolysis of aluminum oxide in the presence of certain electrolytes (such as cryolite, etc.) using a special process. The temperature of electrolytic aluminum liquid is generally between 920 and 950°C, and the residence time in the tank is long. The aluminum liquid is extracted from the electrolytic tank approximately once every 24 hours. Therefore, the following problems are inevitable in electrolytic aluminum liquid:
(1) Slag content. As we all know, the surface of molten aluminum liquid is covered with a layer of α-alumina film. The density of α alumina film is 3.47g/cm3, which is relatively dense and can isolate the molten aluminum liquid from contact with the air, protecting the inside of the melt from further oxidation. However, at the same time, there is also a γ alumina film. The surface of this film is loose, with small pores of Φ5 to 10nm, which is easy to absorb water vapor and contains 1% to 2% H2O at the smelting temperature. As the temperature rises, the amount of water vapor adsorbed decreases, but at 900℃, 0.34% H2O3 is still adsorbed. When the temperature is higher than 900℃, γ-AI2O3 transforms into α-AI2O3, completely dehydrates, and the density increases to 3.97 g/cm3, and the volume shrinks by 13%, thereby destroying the continuity of the alumina film. Its density is greater than 2.3 g/cm3 of molten aluminum, and it becomes slag inclusions when stirred into aluminum liquid. Therefore, the slag inclusion content in electrolytic aluminum liquid is much higher than the slag inclusion content of aluminum ingot remelting aluminum liquid under normal melting conditions.
In fact, in high-temperature electrolytic aluminum liquid, in addition to aluminum oxide slag inclusions, there are also many ions or inclusion particles such as CaF2, MgF2, SiO2.Fe2O3 and surplus Na generated or residual by electrolysis. ,
(2) Gas content. Both electrolytic aluminum liquid and aluminum ingot remelting aluminum liquid contain a certain amount of hydrogen. The amount of hydrogen content is closely related to the melt temperature and the time it stays at high temperature.
1) Relationship between gas content and melt temperature. The relationship between the solubility of hydrogen in aluminum melt and temperature and hydrogen partial pressure can be expressed by the following formula:
![]() (1)
(1)
Where S-hydrogen content in the melt, that is, hydrogen solubility, mL/100g aluminum;
T-aluminum liquid temperature, K;
p-measured hydrogen partial pressure;
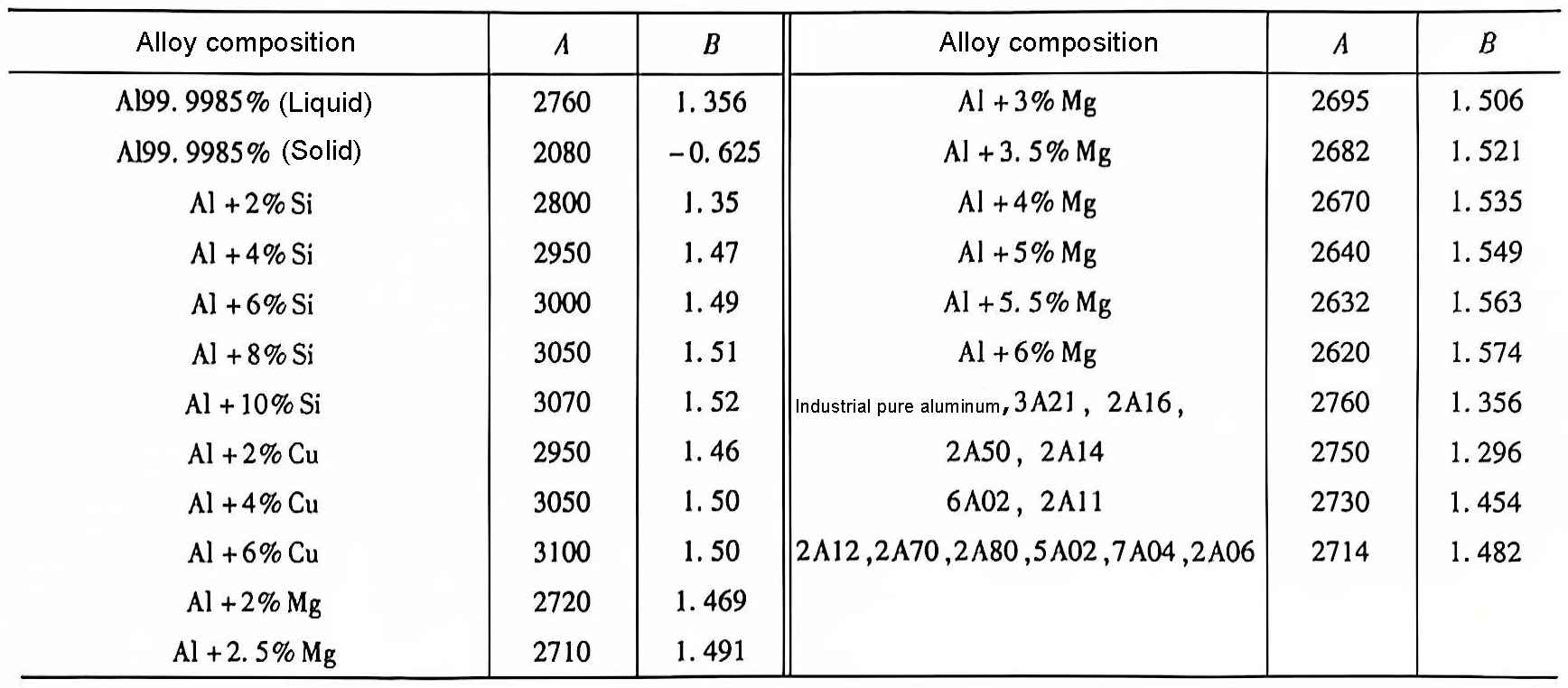
A, B-constants related to the nature of the alloy, their values are shown in Table 1.
Table 1 Constants of aluminum alloy gas dissolution equation
The solubility of hydrogen in pure aluminum melt is shown in Figure 1.
The relationship between the solubility of hydrogen in the melt and the partial pressure of hydrogen can be rewritten as the following expression:
![]()
or
![]() (2)
(2)
where K is a constant;
n is an index (1/2 when the gas is in atomic state and 1 when it is in molecular state).
As can be seen from equation 1 and figure 1, the higher the temperature of the aluminum liquid, the higher the solubility of hydrogen. After the melt exceeds 850℃, the aluminum oxide film of the aluminum liquid is severely damaged, causing the solubility to increase sharply. Under the condition of 900℃, its saturated solubility is far more than 2.2mL/100g aluminum. Therefore, in the production of remelting aluminum ingots, the melt temperature must be strictly controlled. However, when using electrolytic aluminum liquid for direct production, the melt temperature is as high as about 920℃, and it is unrealistic to control the melt temperature of the electrolytic aluminum liquid itself.
2) The relationship between the residence time of the melt and the gas content. It goes without saying that when the aluminum melt stays in the air, before the solubility of hydrogen in it reaches saturation, it always reacts with the water vapor in the air and absorbs hydrogen. Therefore, the longer the melt stays, the more air is absorbed and the higher the gas content (Figure 2).
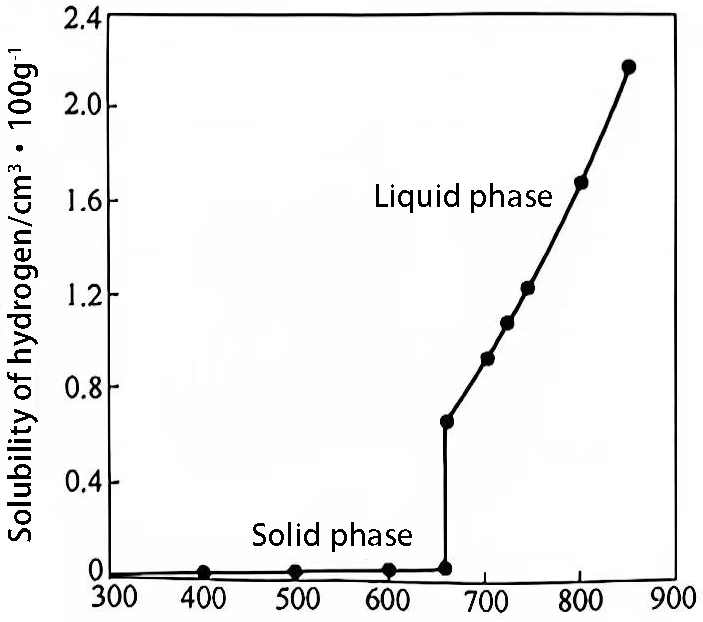
Figure 1 Relationship between the solubility of hydrogen in pure aluminum (99.99%) and temperature
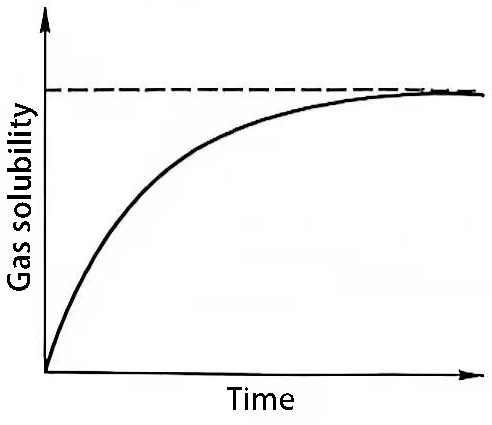
Figure 2 Relationship between the hydrogen content in aluminum melt and the residence time
Practice shows that although the hydrogen content in electrolytic aluminum liquid is far from saturation and is degassed and refined when it leaves the tank, the maximum gas content may still reach 0.3~0.8mL/100g melt when it is transferred to the casting and rolling workshop.
(3) Crystallization core in the melt. Electrolytic aluminum liquid is generated by the electrolysis of alumina and is kept at high temperature for a long time, resulting in a serious overheating state. The active crystallization cores in it are extremely rare. It is generally believed that severely overheated aluminum melt must be cooled and solidified before being melted again to obtain effective active crystallization cores, and normal crystal structure can be obtained after casting or rolling. Directly casting or rolling the overheated high-temperature melt will inevitably produce coarse grain structure, accompanied by various casting or rolling defects. We will discuss this in detail in the following chapters.
2 Research on Optimization of Aluminum Molten Smelting and Casting Technology
The above characteristics of electrolytic aluminum liquid are major obstacles to the production of high-quality plates, strips and foils. Effective technical measures need to be taken to reduce or eliminate their adverse effects. As for the casting and rolling of electrolytic aluminum liquid, the following aspects should be done:
(1) Solid-liquid composition ratio. As mentioned above, high-temperature electrolytic aluminum liquid lacks active crystal cores. It is difficult to obtain qualified and high-quality casting and rolling billets using 100% electrolytic aluminum liquid.
Production and experiments have proved that it is difficult to refine the grains by using aluminum-titanium-boron and aluminum-titanium-carbon for the entire electrolytic aluminum liquid and improving and adjusting the process parameters for casting and rolling; but by adding more than 15% of waste and taking corresponding technical measures in the subsequent process, satisfactory casting and rolling billets can be obtained.
In the production of sheet and strip foil, since the beginning of smelting, the yield is generally between 55% and 80%, and the production waste is about 20% to 45%. Therefore, there is no problem in adding 20% to 40% of solid waste to the electrolytic aluminum liquid. In this way, the high-temperature waste heat of the aluminum liquid can be fully utilized to melt the solid waste. At the same time, burying the waste in the liquid aluminum can reduce the oxidation of the solid waste during the melting process, reduce energy consumption and save resources, improve economic benefits, and reduce environmental pollution.
In the production process, the solid waste or solid aluminum ingots for remelting are first loaded into the hot furnace, so that the solid material can fully absorb the waste heat after pouring the furnace, and the water absorbed on the surface of the waste or aluminum ingot can evaporate. Then inject high-temperature electrolytic aluminum liquid to submerge solid waste or aluminum ingots in molten aluminum liquid as much as possible, so that the solid material can fully absorb the superheated heat of the high-temperature aluminum melt, accelerate the melting of the solid material, quickly reduce the temperature of the superheated melt, and even solidify the superheated melt in the quenching area in contact with the solid material, so as to achieve the purpose of rapid cooling and nucleation; at the same time, improve thermal efficiency, and allow the solid material to melt under the condition of isolation from air to reduce burning loss.
According to the law of conservation of heat, the solid material absorbs heat in the furnace and the temperature rises. When it reaches the melting point of aluminum (660℃), it begins to melt. After melting is completed, it will continue to heat up (700~760℃). The heat required in this process is:
![]() (3)
(3)
Where C1—solid specific heat capacity, take 1.138kJ/(kg·K);
C2——liquid specific heat capacity, take 1.046kJ/(kg·K);
L——melting latent heat, take 393.56kJ/kg;
TM——melting point, ℃.
If 250kg of solid material and 750kg of superheated melt are added, it is calculated based on the ton of melt after mixing. Assume that the aluminum liquid is extracted from the electrolytic cell and transferred to the smelting furnace, and its liquid temperature drops to 880℃. The temperature of the added solid material is 60℃ after preheating. The heat required for the solid material to melt by endothermic absorption is:
250×1.138×(660-60)+250×393.56=269090KJ
The heat that can be released when the melt drops to the solidification point is:
750×1.046×(880-660)=172590kJ
Obviously, under this solid-liquid ratio condition, the heat released by the melt cooling alone is not enough to raise the temperature of the solid material to the melting point. In order to achieve thermal equilibrium between the two objects, the aluminum melt needs to solidify and release the latent heat of crystallization. If it is completely solidified, its latent heat of crystallization is:
750×393.56=295170kJ
The latent heat of crystallization is very large. As long as one-third of the melt crystallizes, the solid material can be heated to 660℃.
The amount of solid material added can be used to control the amount of solidification of the superheated melt during heat exchange. The less solid material added, the less the amount of melt crystallization, and the smaller the probability of cooling and nucleation. On the contrary, the more solid material added, the higher the probability of cooling and nucleation. It can be seen that by controlling the amount of solid material added, the active crystal core of the melt can be controlled to a certain extent.
(2) Temperature control. Generally speaking, when a certain amount of solid material is added to the aluminum melt, its overall temperature will drop to a temperature close to the melting point of aluminum, or even so low that the aluminum melt solidifies. It should be heated immediately. The heating rate can be accelerated as soon as possible without causing local overheating of the aluminum melt, because the burning loss of aluminum is proportional to the melting time (see Figure 3), and the temperature should be controlled between 700 and 760°C. Exceeding 760°C will cause the solid material to melt quickly, and the newly added active crystal cores will be reduced or passivated, which will have an adverse effect on quality.
(3) Chemical composition adjustment. For pure aluminum series, the influence of its iron and silicon content and the ratio between them on the cast and rolled grain size is very important. Under the same process conditions and with the addition of Al-Ti alloy modification, the cast and rolled grain sizes of 1070, 1050 and 1235 alloys are shown in Table 2.
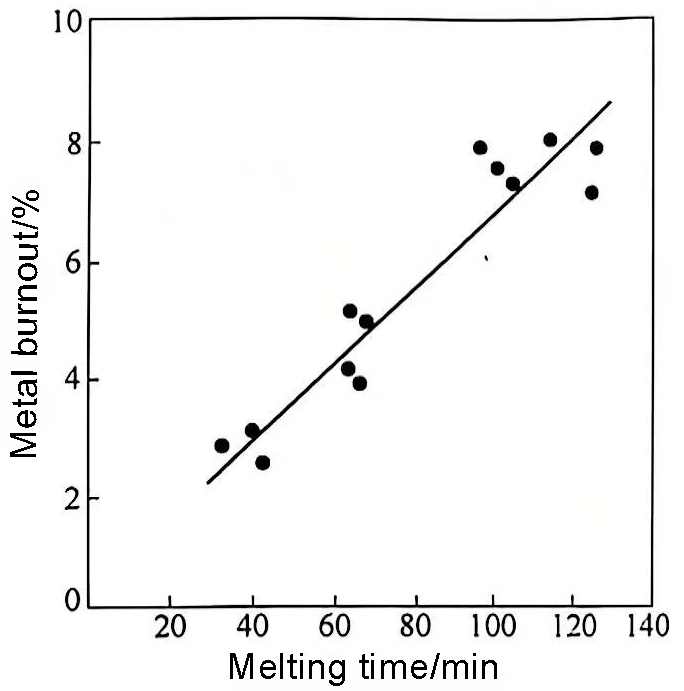
Figure 3 Effect of melting time on metal burnout
Table 2 Cast and rolled grain sizes of 1070, 1050 and 1235 alloys under the same process conditions
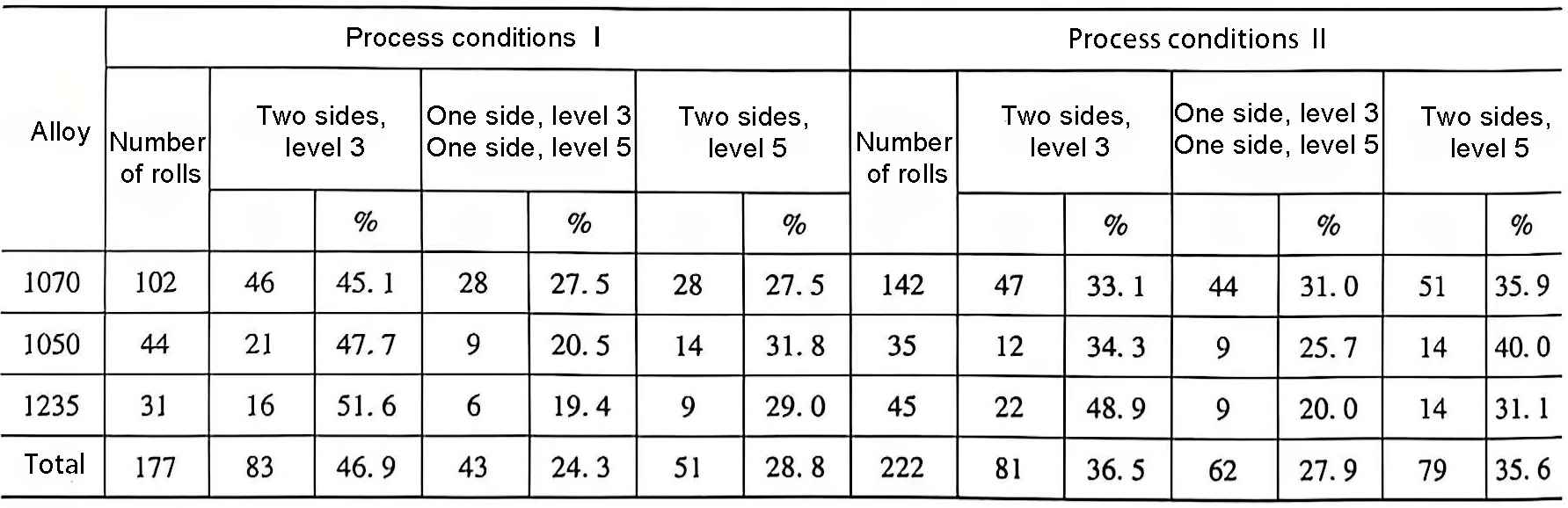
As can be seen from Table 2, with the increase of iron and silicon content, under the same conditions, the grain size tends to be refined; under the same total amount of iron and silicon, the ratio of iron and silicon content is reasonably adjusted, generally Fe/Si is between 2.5 and 4, and the grain size tends to be refined. Therefore, controlling the iron and silicon content and their ratio is one of the effective methods to refine the cast and rolled grains of pure aluminum series
(4) Melt transfer. When pouring electrolytic aluminum liquid into the melting furnace with a ladle or transferring the smelted aluminum liquid into the static furnace, special attention should be paid to the melt drop during the process. The large drop will cause a great impact when the melt falls. In this way, first, it is easy to entrain gas and increase the gas content of the melt; second, the high-temperature melt reacts with the air to produce a large amount of oxidized slag, which not only pollutes the melt but also reduces the production yield. Therefore, the general method is to reduce the drop as much as possible, or use a vertical round tube during the melt transfer process. The round tube is close to the bottom of the furnace, so that the melt flows in the vertical round tube. In the process of falling and passing through the compressed air, the melt is prevented from contacting with the air to produce oxidation and entrainment of gas. Although this method is effective, it cannot fundamentally solve the problem of entrainment and oxidation. The most effective method is to use compressed air drainage, siphon closed transfer, and spray nitrogen chlorine (argon chlorine) to refine aluminum melt, which can completely prevent entrainment and oxidation during transfer.
The principle of the transfer device is shown in Figure 4.
As shown in the figure, one end of the siphon is inserted into the smelting furnace (or the melt ladle), and the other end is placed in the static furnace (or the melting furnace). In addition, an air duct is connected to the air duct 4, and valves 1 and 2 are installed. Valve 1 is connected to compressed air. Another air duct is connected between valves 1 and 2, and valve 3 is installed to connect nitrogen, chlorine or argon chlorine gas.
Figure 4 Schematic diagram of siphon and refining device
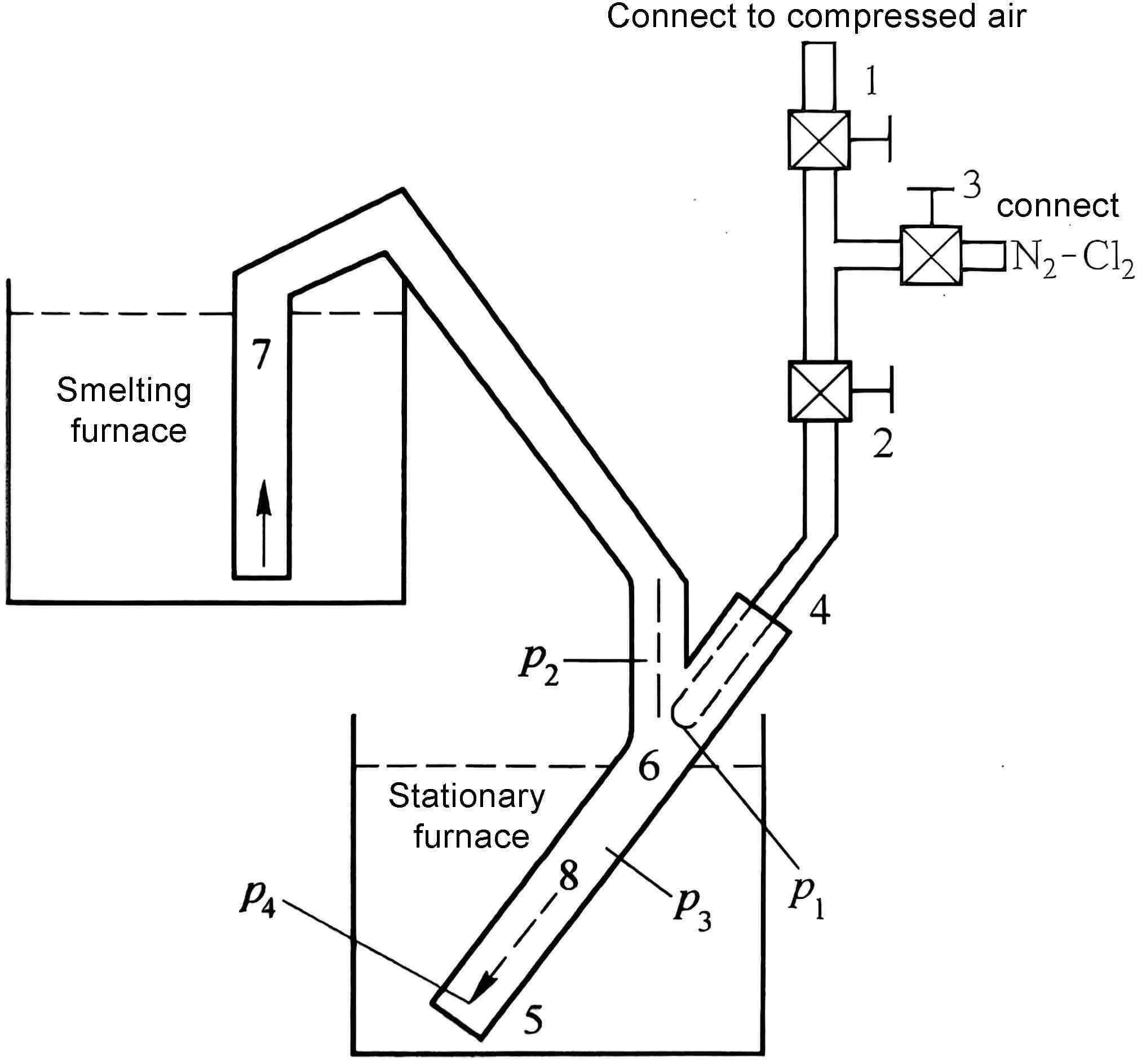
The principle and pressure head of siphon generation: Valve 3 is closed, valves 1 and 2 are opened, and compressed air flows into the siphon through air duct 4, and a jet is generated at 6, converting the pressure of the compressed air into the kinetic energy of the jet. That is, because the compressed air has a high speed at 6, reaching the speed of sound, a negative pressure is generated at pipe 7, forming a suction force.
Assume G1-G3 are the mass flow rates of the injection fluid, the conveying fluid, and the mixed fluid respectively; Q1-Q3 are the volume flow rates of the injection fluid, the conveying fluid, and the mixed fluid respectively; P1-P3 are the densities of the injection fluid, the conveying fluid, and the mixed fluid respectively; u1-u3 are the flow rates of the injection fluid, the conveying fluid, and the mixed fluid respectively; A1-A3 are the cross-sectional areas of the injection fluid, the conveying fluid, and the mixed fluid respectively; according to the gas flow continuity equation:
![]() (4)
(4)
or
![]() (5)
(5)
Therefore, the density of the mixed fluid is:
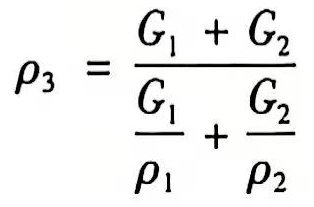 (6)
(6)
If the friction resistance in the ejector is ignored, its momentum equation is:
![]() (7)
(7)
In the formula, p1 – injection fluid pressure;
p3 – mixed fluid pressure.
Assume that the gas pressure at the outlet end 5 of the siphon is P4, then:
![]() (8)
(8)
In the formula, φ is the pressure recovery coefficient of the expansion section. The pressure generated by the ejector is:
![]() (9)
(9)
In this device, before the aluminum melt passes through, the negative pressure generated by the injection fluid only plays a role in exhausting air, so h is the suction force of the ejector. In practice, if the inner diameter of the siphon is Φ52mm and the compressed air pressure (gauge pressure) is 6.7kgf/cm2, the suction force (i.e. negative pressure) generated is 370mmHg, which is converted into the suction force on the aluminum melt:
![]()
Experiments have proved that this data is correct. In this way, the aluminum melt is transferred from the smelting furnace to the static furnace, and it runs in a fully enclosed pipeline. It does not come into contact with air during the transfer, which can completely avoid oxidation, slag formation and air entrainment, and effectively improve the transfer quality. If nitrogen chlorine (or argon chlorine) refining is introduced (this point will be discussed in detail in the refining section later), the refining quality can be further improved, and refining while transferring can be achieved, reducing the subsequent refining time.