Graphene supercapacitors is a special kind of capacitor with unusually high conductivity and large surface area, which is superior to similar products in the process of energy storage and release.
 As one of the most abundant elements on earth, carbon plays a vital role in human life and social development. Located in group ⅣA in the second period of the periodic table, the outermost four valence electrons can form single bonds by sp3 hybridization, double bonds by sp2 hybridization and triple bonds by sp hybridization. Various bonding properties give carbon diverse existence forms.
As one of the most abundant elements on earth, carbon plays a vital role in human life and social development. Located in group ⅣA in the second period of the periodic table, the outermost four valence electrons can form single bonds by sp3 hybridization, double bonds by sp2 hybridization and triple bonds by sp hybridization. Various bonding properties give carbon diverse existence forms.
In terms of simple matter, carbon allotropes include three-dimensional diamond, two-dimensional graphite lamellae and graphene, one-dimensional carbine and carbon nanotubes, zero-dimensional fullerenes and quantum dots, and other forms with completely different structures and properties.
 Carbon materials have various structures and different properties. It can be said that carbon materials almost include the characteristics of all substances on the earth, which makes carbon materials widely used. Among the electrode materials for supercapacitors, the earliest and most mature research technology is carbon materials.
Carbon materials have various structures and different properties. It can be said that carbon materials almost include the characteristics of all substances on the earth, which makes carbon materials widely used. Among the electrode materials for supercapacitors, the earliest and most mature research technology is carbon materials.
Its research started from the relevant patent published by Beck in 1957, and its development has been more than 60 years. The reason why carbon materials become the first choice for the preparation of supercapacitor electrodes is that they usually have the following characteristics: (1) chemically inert, stable in various acid and alkali solutions, and do not react with electrodes; (2) Large specific surface area, well-developed pore structure and high porosity, which can adsorb a large amount of electrolyte solution;(3)High purity, good conductivity, small leakage current; (4) High thermal stability, stable performance in a wide temperature range; (5)Еasy to process into various shapes of electrodes; (6)The price is low and the source is abundant.
 At present, the most studied electrode materials for supercapacitors mainly include activated carbon, active carbon fiber, carbon aerogel, carbon nanotube and graphene.
At present, the most studied electrode materials for supercapacitors mainly include activated carbon, active carbon fiber, carbon aerogel, carbon nanotube and graphene.
Activated charcoal
Activated carbon The industrial production and application of activated carbon has a long historyDue to its rich pore structure and huge specific surface area, it has strong adsorption and catalytic properties, and is widely used in environmental protection, chemical industry, energy, medicine and other fields. Activated carbon is the earliest carbon electrode material used in supercapacitors.
1 The structure of activated carbon-Graphene supercapacitors
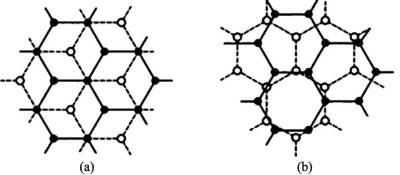
 Structure of activated carbon Activated carbon is a kind of amorphous carbon, which is composed of graphitized activated carbon microcrystal and non-graphitized amorphous carbon. The graphite microcrystal, which is similar to the two-dimensional structure of graphite, is a planar network structure formed by the hexagonal ring formed by sp2 mixed with hybrid carbon. These graphite microcrystals have a small particle size (about 1 ~ 3nm) and are arranged in an irregular and loose arrangement, which is referred to as “spiral layer structure” or “chaotic layer structure”, as shown in Figure 2-1.
Structure of activated carbon Activated carbon is a kind of amorphous carbon, which is composed of graphitized activated carbon microcrystal and non-graphitized amorphous carbon. The graphite microcrystal, which is similar to the two-dimensional structure of graphite, is a planar network structure formed by the hexagonal ring formed by sp2 mixed with hybrid carbon. These graphite microcrystals have a small particle size (about 1 ~ 3nm) and are arranged in an irregular and loose arrangement, which is referred to as “spiral layer structure” or “chaotic layer structure”, as shown in Figure 2-1.
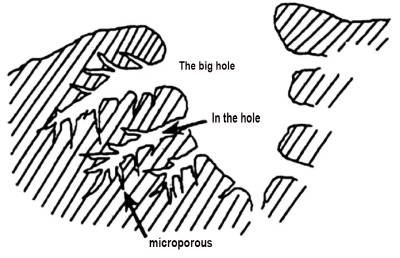
Because the arrangement of graphite microcrystals is irregular and disordered, the pores of different sizes and shapes are formed between microcrystals and amorphous carbonites and between microcrystals, including open pore shape, semi-obturator pore shape and mesenchymal cage shape. According to the size of pores, it can be divided into: large pores (pore diameter greater than 50nm) are called supply or transport pores, accounting for a small proportion, weak adsorption performance, mainly play a role in providing channels for the entry of adsorbent molecules, which plays an important role in the adsorption speed; Mesopore (pore diameter between 2 and 50nm) is also called mesopore.
 On the one hand, mesopore has the same function as macropore, which can act as a channel for adsorbent molecules to enter the micropore. On the other hand, mesopore can adsorb macromolecular substances that cannot enter the micropore. Micropores (pore diameter is less than 2nm), also known as adsorption pores, activated carbon more than 90% of the specific surface area and pore capacity are from the contribution of micropores, micropores for gas and liquid small molecules have a strong adsorption effect, to a large extent determines the adsorption performance of the entire activated carbon. The three kinds of pores are staggered and crossed to form a tree structure, as shown in Figure 2-2.
On the one hand, mesopore has the same function as macropore, which can act as a channel for adsorbent molecules to enter the micropore. On the other hand, mesopore can adsorb macromolecular substances that cannot enter the micropore. Micropores (pore diameter is less than 2nm), also known as adsorption pores, activated carbon more than 90% of the specific surface area and pore capacity are from the contribution of micropores, micropores for gas and liquid small molecules have a strong adsorption effect, to a large extent determines the adsorption performance of the entire activated carbon. The three kinds of pores are staggered and crossed to form a tree structure, as shown in Figure 2-2.
In addition, the pore size structure of activated carbon has different relationship with the molecular scale of adsorbent, and the adsorption state is also different. When the pore size of activated carbon is much smaller than the molecular diameter of adsorbent,Molecules can not enter the pore, activated carbon does not adsorption; When the pore size of activated carbon and adsorbent molecular diameter is similar, activated carbon on the molecular adsorption capture ability is the strongest, even if the concentration of low molecules can also be adsorbed; When the pore size of activated carbon is larger than the molecular diameter of adsorbent, the molecular capillary condensation occurs in the pore, which can increase the adsorption capacity. When the pore size of activated carbon is much larger than the adsorbent diameter, the molecules are easy to be absorbed, but also easy to occur desorption, so that the final adsorption amount is small. Therefore, only when the pore size of activated carbon and adsorbent molecules match each other can the adsorption process be carried out effectively.
2 Performance characteristics of activated carbon
 Activated carbon, as the earliest and most widely used electrode material for supercapacitors, has the following performance advantages. (1)Large specific surface area, the theoretical specific surface area of activated carbon is 500 ~ 3000m2·g-1, which is made into a supercapacitor electrode. The theoretical specific capacitance of a single electrode can be as high as 500F·g-1, but the actual specific capacitance is far less than this A value. (2)The pore structure is developed, and the number of pores is about 1020 ·g-1, which can absorb a large number of electrolyte solution molecules. (3) High chemical stability, no chemical reactions, not easy to be acid, alkali and other solutions corrosion. (4) High purity, good electrical conductivity, good thermal stability. (5)Easy to process, good compatibility with other materials. (6)The price is low and the source is abundant.
Activated carbon, as the earliest and most widely used electrode material for supercapacitors, has the following performance advantages. (1)Large specific surface area, the theoretical specific surface area of activated carbon is 500 ~ 3000m2·g-1, which is made into a supercapacitor electrode. The theoretical specific capacitance of a single electrode can be as high as 500F·g-1, but the actual specific capacitance is far less than this A value. (2)The pore structure is developed, and the number of pores is about 1020 ·g-1, which can absorb a large number of electrolyte solution molecules. (3) High chemical stability, no chemical reactions, not easy to be acid, alkali and other solutions corrosion. (4) High purity, good electrical conductivity, good thermal stability. (5)Easy to process, good compatibility with other materials. (6)The price is low and the source is abundant.
3 Preparation of activated carbon
3.1 Prepare raw materials
 Raw materials for the preparation of activated carbon There are abundant sources of raw materials for the preparation of activated carbon. Generally, as long as the materials rich in carbon can be used as raw materials for the preparation of activated carbon. According to the source of raw materials can be divided into plant raw materials and mineral raw materials two categories. Among them, plants have a wide range of raw materials, in addition to traditional wood,Coconut shell, walnut shell, apricot kernel, olive kernel, rice husk, etc., as well as agricultural and forestry by-products and waste living carbon, such as wood chips, bark, bamboo, cotton stalk, peanut shell, waste plastic, urban garbage, etc. At present, it is generally believed that the fruit shell is the best raw material for the preparation of activated carbon, which has high strength and very fine micropores, but the fruit shell resources are limited and not easy to concentrate storage. Mineral raw materials include coal measures raw materials and petroleum raw materials. Due to the rich, cheap and easy availability of coal resources, the coal measures raw material is the main raw material for preparing activated carbon in a long period of time. Petroleum raw materials mainly refer to carbon-containing products and wastes in the process of petroleum refining, such as asphalt, oil residue, petroleum coke, etc. Petroleum coke has the advantages of high carbon content, low ash content and good electrical conductivity, which is suitable for the preparation of activated carbon raw materials.
Raw materials for the preparation of activated carbon There are abundant sources of raw materials for the preparation of activated carbon. Generally, as long as the materials rich in carbon can be used as raw materials for the preparation of activated carbon. According to the source of raw materials can be divided into plant raw materials and mineral raw materials two categories. Among them, plants have a wide range of raw materials, in addition to traditional wood,Coconut shell, walnut shell, apricot kernel, olive kernel, rice husk, etc., as well as agricultural and forestry by-products and waste living carbon, such as wood chips, bark, bamboo, cotton stalk, peanut shell, waste plastic, urban garbage, etc. At present, it is generally believed that the fruit shell is the best raw material for the preparation of activated carbon, which has high strength and very fine micropores, but the fruit shell resources are limited and not easy to concentrate storage. Mineral raw materials include coal measures raw materials and petroleum raw materials. Due to the rich, cheap and easy availability of coal resources, the coal measures raw material is the main raw material for preparing activated carbon in a long period of time. Petroleum raw materials mainly refer to carbon-containing products and wastes in the process of petroleum refining, such as asphalt, oil residue, petroleum coke, etc. Petroleum coke has the advantages of high carbon content, low ash content and good electrical conductivity, which is suitable for the preparation of activated carbon raw materials.
3.2 Preparation Methods
The preparation of activated carbon is generally divided into two steps: carbonization and activation. Carbonization refers to the heating of raw materials to a certain extent under conditions of air isolation or protection from inert gasesTemperature, so that the volatile non-carbon components in the raw material decomposition discharge. The whole process can be divided into four stages according to the temperature change:
 The first stage is the drying process, the temperature is 120 ~ 150℃, mainly to remove the water evaporation in the raw material, the temperature is not high, the chemical composition of the raw material does not change; The second stage is the pre-carbonization process, when the temperature rises to 150 ~ 275℃, the thermal decomposition of raw materials is obvious, the chemical composition of materials begins to change, the internal structure recombines, and some unstable components begin to decompose. The third stage is the carbonization process, which is the key link of the carbonization of activated carbon. The reaction temperature reaches about 400℃, and the rapid decomposition of raw materials produces a large number of gases and liquids. The fourth stage is calcination process, the system temperature reaches 500℃, the raw material is further calcined, a small amount of residual volatile substances are discharged, and the carbon material with increased fixed carbon content is obtained. The essence of carbonization is the process of thermal decomposition and thermal condensation of organic matter in raw materials, among which the carbonization temperature, carbonization time, heating rate and other parameters are important factors affecting the quality of carbonization products, and will also have a certain impact on the subsequent activation process.
The first stage is the drying process, the temperature is 120 ~ 150℃, mainly to remove the water evaporation in the raw material, the temperature is not high, the chemical composition of the raw material does not change; The second stage is the pre-carbonization process, when the temperature rises to 150 ~ 275℃, the thermal decomposition of raw materials is obvious, the chemical composition of materials begins to change, the internal structure recombines, and some unstable components begin to decompose. The third stage is the carbonization process, which is the key link of the carbonization of activated carbon. The reaction temperature reaches about 400℃, and the rapid decomposition of raw materials produces a large number of gases and liquids. The fourth stage is calcination process, the system temperature reaches 500℃, the raw material is further calcined, a small amount of residual volatile substances are discharged, and the carbon material with increased fixed carbon content is obtained. The essence of carbonization is the process of thermal decomposition and thermal condensation of organic matter in raw materials, among which the carbonization temperature, carbonization time, heating rate and other parameters are important factors affecting the quality of carbonization products, and will also have a certain impact on the subsequent activation process.
Activation process is the most critical step in the preparation of activated carbon, which can effectively regulate the specific surface area and pore structure of activated carbon. The activation process is a complex chemical reaction between activator and carbon material. This process can be divided into three main stages: the first stage is at high temperature, the initial pores blocked by disordered carbon atoms and heteroatoms under the action of activator are opened and further expanded, which is called transverse pore expansion; In the second stage, the unsaturated carbon atoms at the edge of the newly opened pores further react with the activator, making the pores develop continuously to the depth, and achieve the merger or connection between pores. In the third stage, new unsaturated carbon atoms or active spots are exposed to the microcrystalline surface, and the uneven combustion of the microcrystalline surface leads to the formation of a large number of new pores. Changing the temperature, time, gaseous environment and other conditions of activation reaction can regulate the porosity, pore size distribution and inner surface properties of activated carbon to a certain extent.
 At present, the commonly used activation methods include physical activation method, chemical activation method, physical-chemical combined activation method and other activation methods.
At present, the commonly used activation methods include physical activation method, chemical activation method, physical-chemical combined activation method and other activation methods.
(1) Physical activation method physical activation, also known asFor gas activation or thermal activation, carbon materials and water vapor, carbon dioxide, oxygen, air and other gases with oxidation characteristics are heated at a high temperature of 600 ~ 1200℃ for activation. Its essence is the oxidation reaction between the unsaturated carbon atoms in the raw material located at the corner of the microcrystal or the defect of the base surface and the oxidizing gas, which eliminates the residual volatile pyrolysis products in the carbon material and greatly increases the pore volume and the specific surface area of the obtained activated carbon material.
 The main advantages of the physical activation method are simple production process, less pollution, and the product can be directly used without cleaning. However, the activation temperature of the method is higher, the activation time is longer, the energy consumption is high, and the activated carbon pore is not developed enough, and the specific surface area is low.
The main advantages of the physical activation method are simple production process, less pollution, and the product can be directly used without cleaning. However, the activation temperature of the method is higher, the activation time is longer, the energy consumption is high, and the activated carbon pore is not developed enough, and the specific surface area is low.
(2) Chemical activation method Chemical activation method is a method of mixing thick solutions containing carbon raw materials and chemical reagents, stirring them evenly, heating up, pyrolysis, cooling, and continuous washing with detergent to remove activators. FIG. 2-3 shows its technological process. Its essence is through the dehydration, expansion and skeleton of chemical reagents on raw materials, so that the two are gas activation or thermal activation. It is the method of heating carbon materials and water vapor, carbon dioxide, oxygen, air and other gases with oxidation characteristics at the high temperature of 600 ~ 1200℃ for activation. Its essence is the oxidation reaction between the unsaturated carbon atoms in the raw material located at the corner of the microcrystal or the defect of the base surface and the oxidizing gas, which eliminates the residual volatile pyrolysis products in the carbon material and greatly increases the pore volume and the specific surface area of the obtained activated carbon material. The main advantages of the physical activation method are simple production process, less pollution, and the product can be directly used without cleaning. However, the activation temperature of the method is higher, the activation time is longer, the energy consumption is high, and the activated carbon pore is not developed enough, and the specific surface area is low.
 (3) Chemical activation method Chemical activation method is a method of mixing thick solutions containing carbon raw materials and chemical reagents, stirring them evenly, heating up, pyrolysis, cooling, and continuous washing with detergent to remove activators. FIG. 2-3 shows its technological process. Its essence is through the chemical reagents to the raw material dehydration, moistening swelling and skeleton action, so that bothA series of polycondensation and cross-linking reactions take place, thereby releasing part of the carbon atoms in the raw material, and at the same time releasing hydrogen and oxygen in the form of water vapor, forming a large number of pores.
(3) Chemical activation method Chemical activation method is a method of mixing thick solutions containing carbon raw materials and chemical reagents, stirring them evenly, heating up, pyrolysis, cooling, and continuous washing with detergent to remove activators. FIG. 2-3 shows its technological process. Its essence is through the chemical reagents to the raw material dehydration, moistening swelling and skeleton action, so that bothA series of polycondensation and cross-linking reactions take place, thereby releasing part of the carbon atoms in the raw material, and at the same time releasing hydrogen and oxygen in the form of water vapor, forming a large number of pores.
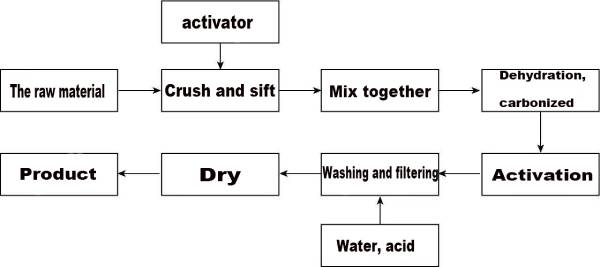
In the chemical activation method, common activators are zinc chloride (ZnCl2), phosphoric acid (H3PO4), potassium hydroxide (KOH), sodium hydroxide (NaOH), calcium chloride (CaCl2) and so on. The action process of these activators on carbon raw materials is different, but the action mechanism is similar. Through the addition of activators, part of carbon, hydrogen and oxygen contained in the raw materials are decomposed and separated in the form of carbon dioxide, carbon monoxide and water vapor, and the carbonization temperature is significantly reduced at the same time.
 (1)ZnCl2 activation method ZnCl2 activation method is one of the earliest chemical activation methods for the preparation of activated carbon, its strong dehydration effect makes the activation temperature significantly reduced, generally in 500 ~ 700℃. The activation mechanism is that ZnCl2, as a Lewis acid, interacts with oxygen-containing functional groups, releasing hydrogen and oxygen in the carbon raw material in the form of water vapor, leading to the aromatization of carbon chains to form pore structures, and changing the thermal decomposition process of raw materials to inhibit the generation of tar. Due to the reaction temperature lower than 700℃, ZnCl2 in the form of liquid evenly distributed in the activated carbon, when the water to remove ZnCl2 washing, the formation of developed micro pores, but also caused the removal of ZnCl2 consumption, high activation cost, and pollution to the environment.
(1)ZnCl2 activation method ZnCl2 activation method is one of the earliest chemical activation methods for the preparation of activated carbon, its strong dehydration effect makes the activation temperature significantly reduced, generally in 500 ~ 700℃. The activation mechanism is that ZnCl2, as a Lewis acid, interacts with oxygen-containing functional groups, releasing hydrogen and oxygen in the carbon raw material in the form of water vapor, leading to the aromatization of carbon chains to form pore structures, and changing the thermal decomposition process of raw materials to inhibit the generation of tar. Due to the reaction temperature lower than 700℃, ZnCl2 in the form of liquid evenly distributed in the activated carbon, when the water to remove ZnCl2 washing, the formation of developed micro pores, but also caused the removal of ZnCl2 consumption, high activation cost, and pollution to the environment.
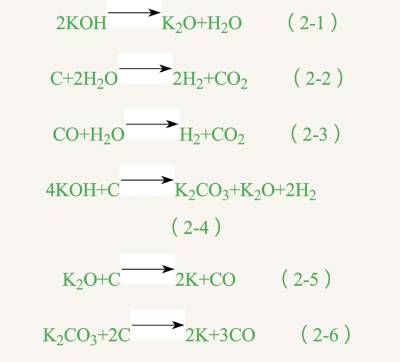
(2) KOH activation method KOH is one of the most representative alkali activators, AMOCO research found that adding KOH to coal or petroleum coke, activation can be obtained after the high specific surface area of 2500m2·g-1 activated carbon. The activated carbon product obtained by this methodThe distribution of micropores is concentrated, the pore structure is uniform and developed, and the specific surface area is large, which has attracted a lot of attention from scholars at home and abroad in recent years. The specific process is to add alkali to the raw material in accordance with a certain proportion of mixing, after grinding and mixing evenly, in inert gas or closed system heating to 700 ~ 800℃ carbonization, activation, that is, to get a large number of cage-like microporous structure of activated carbon. According to the temperature of the system, the whole activation process is divided into four stages [1] : in the first stage, low temperature dehydration (<300℃), the attached water on the surface of the raw material and the combined water generated by the reaction overflow in the form of steam; The second stage, pre-activation (300 ~ 500℃), the production of water vapor and carbon dioxide, carbon monoxide and other gases and volatilization; In the third stage, the molecules are activated at moderate temperature (500 ~ 600℃), crosslinking or polycondensation reaction occurs, and some non-carbon elements are volatilized. In the fourth stage, KOH is almost completely transformed into K2CO3 and K2O by high temperature activation (>600℃). These two compounds are further reacted with carbon materials to generate highly active potassium. When the temperature is higher than 762℃, potassium diffuses in gas state to form vertical The final activated carbon product has a large number of micropores and high specific surface area. The main chemical reactions that occur during the whole process are as follows:
 In the process of KOH activation, there are many factors that affect the performance of products, and the main factors are as follows.
In the process of KOH activation, there are many factors that affect the performance of products, and the main factors are as follows.
a. Agent to material ratio: that is, the selection of activator KOH and carbon containing raw material between the mass ratio, it on activated carbon products Performance has a significant impact. When the ratio of KOH to carbon material is low, the activation reaction is not sufficient, and the products formed have fewer pores and lower specific surface area.When the alkali-carbon ratio is too large, the excess KOH will cause the excessive reaction of activated carbon, so that some of the formed better pores collapse into series of large pores, so that the product’s specific surface area and pore volume are reduced. Therefore, according to the material and particle size of carbon raw materials and specific process to choose the appropriate ratio of agent to material.
 b. Particle size of raw material: particle size of raw material directly affects the full degree of contact between raw material and activator. Under the same conditions, the smaller the particle size of the raw material, the more developed the pore structure of the product, but too small the particle size will bring difficulties to sample preparation and filtration, and affect the yield.
b. Particle size of raw material: particle size of raw material directly affects the full degree of contact between raw material and activator. Under the same conditions, the smaller the particle size of the raw material, the more developed the pore structure of the product, but too small the particle size will bring difficulties to sample preparation and filtration, and affect the yield.
c. Addition method of KOH: The addition method of KOH mainly includes simple incorporation method and impregnation method. The simple mixing method is to mix KOH powder and carbon raw material, which is simple to operate but has low yield, small product specific surface area and pore capacity. The impregnation method starts with KOH After a certain concentration of solution is prepared, the carbon raw material is added to the solution, and KOH is adsorbed to the surface and internal pores of the raw material through impregnation. This addition method can make the activator contact with the carbon material more fully, with a low loss rate and a larger specific surface area of the product.
 d. Activation temperature: Theoretically, the higher the temperature, the higher the molecular activation energy and the higher the reaction degree, the larger the specific surface area of the product, and the phenomenon of pore expansion will also occur, but the yield will also be reduced.
d. Activation temperature: Theoretically, the higher the temperature, the higher the molecular activation energy and the higher the reaction degree, the larger the specific surface area of the product, and the phenomenon of pore expansion will also occur, but the yield will also be reduced.
e. activation time: some conditions, as the activation time of rights, product yield decreased, and the specific surface area, pore volume increased, but when the activation time after reaching a certain value, due to the reaction of generated microporous was further collapse into the holes or big hole, no longer increases the specific surface area and pore volume, it has reduced. In addition, the operation of KOH active agent washing and product drying at the end of the process also has a great influence on the performance of activated carbon. The specific surface area of activated carbon prepared by alkaline activation method is relatively high Large, short activation time, more mature process; However, alkali itself is corrosive to equipment, difficult to recover, in addition to the high activation temperature, energy consumption and other shortcomings, so there are still many problems in large-scale industrial production.
 (3) H3PO4 activation method H3PO4 activation method is one of the most commonly used methods for the preparation of activated carbon because of its light pollution to the environment and low production cost. Its activation mechanism is similar to that of ZnCl2. H3PO4 plays the following roles in the activation process.
(3) H3PO4 activation method H3PO4 activation method is one of the most commonly used methods for the preparation of activated carbon because of its light pollution to the environment and low production cost. Its activation mechanism is similar to that of ZnCl2. H3PO4 plays the following roles in the activation process.
a. Dehydration: in the absence of H3PO4, most of the hydrogen and oxygen volatilized by organic matter, while in the form of water vapor removal under the action of H3PO4, more carbon can be retained and the yield increased.
 b. Swelling effect: at low temperature, H3PO4 penetrates into the raw material, accelerates the swelling and dissolution of cellulose and lignin in the raw material through ionization, and promotes subsequent hydrolysis and oxidation reactions.
b. Swelling effect: at low temperature, H3PO4 penetrates into the raw material, accelerates the swelling and dissolution of cellulose and lignin in the raw material through ionization, and promotes subsequent hydrolysis and oxidation reactions.
 c. Oxidation: H3PO4 has certain oxidation ability. Above 200℃, H3PO4 forms pyrophosphate network structure through cross-linking polycondensation reaction. Pyrophosphate has strong corrosion and oxidation properties, and further oxidizes carbon materials to form more micropores and mesoporous materials.
c. Oxidation: H3PO4 has certain oxidation ability. Above 200℃, H3PO4 forms pyrophosphate network structure through cross-linking polycondensation reaction. Pyrophosphate has strong corrosion and oxidation properties, and further oxidizes carbon materials to form more micropores and mesoporous materials.
 d. Aromatic condensation: When the temperature continues to rise, polycondensation carbon structure is formed by polycondensation, cyclization and cross-linking of phospholipid bond with organic matter or other polymers, which can be activated and transformed into random layer microcrystalline structure of carbon at appropriate temperature.
d. Aromatic condensation: When the temperature continues to rise, polycondensation carbon structure is formed by polycondensation, cyclization and cross-linking of phospholipid bond with organic matter or other polymers, which can be activated and transformed into random layer microcrystalline structure of carbon at appropriate temperature.
In short, in the whole activation process, H3PO4 can promote the pyrolysis reaction process, reduce the activation temperature, prevent the particle shrinkage under high temperature conditions, reduce the formation of tar. After washing and removing phosphate, activated carbon products with developed pore structure can be obtained. The products prepared by phosphoric acid activation method have wide pore size distribution and well-developed mesoporous pores. Phosphoric acid itself has low corrosion and less environmental pollution. The activated carbon produced is uniform and stable.Good settling performance, wide application areas.
 (3) physical-chemical combined activation method Physical-chemical combined activation method is to combine the advantages of physical activation and chemical activation, the first use of simple physical activation and chemical activation of the secondary activation method. However, this method still cannot overcome some adverse factors, and additional steps are added. At present, the composite activation technology of chemical impregnation and physical activation is mostly adopted. The activated carbon materials with excellent adsorption performance and reasonable pore size distribution can be prepared by controlling factors such as the quality ratio of the activator raw material, impregnation time, activation temperature and activation time. Hu et al. impregnated with ZnCl2 and activated coconut husk with CO2 at high temperature to prepare a series of mesoporous activated carbon with controllable pore structure. Zhang Wenhui et al. took coal-based carbon source as raw material, impregnated the sample with KOH and activated it with water vapor, and obtained the product with a specific surface area greater than 1500m2·g-1 and good adsorption performance in a relatively short time.
(3) physical-chemical combined activation method Physical-chemical combined activation method is to combine the advantages of physical activation and chemical activation, the first use of simple physical activation and chemical activation of the secondary activation method. However, this method still cannot overcome some adverse factors, and additional steps are added. At present, the composite activation technology of chemical impregnation and physical activation is mostly adopted. The activated carbon materials with excellent adsorption performance and reasonable pore size distribution can be prepared by controlling factors such as the quality ratio of the activator raw material, impregnation time, activation temperature and activation time. Hu et al. impregnated with ZnCl2 and activated coconut husk with CO2 at high temperature to prepare a series of mesoporous activated carbon with controllable pore structure. Zhang Wenhui et al. took coal-based carbon source as raw material, impregnated the sample with KOH and activated it with water vapor, and obtained the product with a specific surface area greater than 1500m2·g-1 and good adsorption performance in a relatively short time.
4 Activated carbon modification
 With the increasingly high performance requirements for carbon-based materials, simple carbonization, activation process has been difficult to meet, therefore, the late regulation and modification of activated carbon technology has been paid more and more attention. The modification of activated carbon includes two aspects: the first is the surface structure modification, which refers to the physical or chemical method to increase the specific surface area of activated carbon material and adjust the pore structure and distribution of activated carbon in the preparation process, so that the pore structure of activated carbon is changed, so as to change its adsorption and energy storage performance. Second, the surface chemical properties of activated carbon modification, is through a certain method to change the type and number of functional groups on the surface, the surface of the heteroatom and its surrounding atmosphere structure, so that the active site increase, so as to control its binding ability with the adsorbed. At present, the research on the late preparation technology of activated carbon modification and modification has attracted much attention. According to the different principles and characteristics of the technical treatment basis, the modification technology can be divided into the following types.
With the increasingly high performance requirements for carbon-based materials, simple carbonization, activation process has been difficult to meet, therefore, the late regulation and modification of activated carbon technology has been paid more and more attention. The modification of activated carbon includes two aspects: the first is the surface structure modification, which refers to the physical or chemical method to increase the specific surface area of activated carbon material and adjust the pore structure and distribution of activated carbon in the preparation process, so that the pore structure of activated carbon is changed, so as to change its adsorption and energy storage performance. Second, the surface chemical properties of activated carbon modification, is through a certain method to change the type and number of functional groups on the surface, the surface of the heteroatom and its surrounding atmosphere structure, so that the active site increase, so as to control its binding ability with the adsorbed. At present, the research on the late preparation technology of activated carbon modification and modification has attracted much attention. According to the different principles and characteristics of the technical treatment basis, the modification technology can be divided into the following types.
4.1 Heat treatment method
 Heat treatment refers to the process of heating activated carbon at high temperature under certain conditions. Through heat treatment, the initial pore size, pore volume and functional groups on the surface of the original carbon material can be changed, so as to obtain the activated carbon material with developed pores, low oxygen and oxidation resistance. Kim heat-treated activated carbon in high temperature and nitrogen environment to obtain activated carbon containing pyrrole nitrogen on the surface, which greatly improved the hydrophilicity and wettability of activated carbon. In addition to the ordinary heating method, the method of microwave heating modified activated carbon has many advantages, and has been paid more and more attention by researchers. Microwave heating mainly causes the shrinkage of carbon skeleton through rapid and efficient thermal action, which leads to the change of pore size and pore volume. In addition, the use of microwave heat treatment of activated carbon under different atmospheres will affect the properties of its surface groups, such as oxidizing atmosphere is conducive to the formation of acidic groups, reducing atmosphere is conducive to the formation of basic groups.
Heat treatment refers to the process of heating activated carbon at high temperature under certain conditions. Through heat treatment, the initial pore size, pore volume and functional groups on the surface of the original carbon material can be changed, so as to obtain the activated carbon material with developed pores, low oxygen and oxidation resistance. Kim heat-treated activated carbon in high temperature and nitrogen environment to obtain activated carbon containing pyrrole nitrogen on the surface, which greatly improved the hydrophilicity and wettability of activated carbon. In addition to the ordinary heating method, the method of microwave heating modified activated carbon has many advantages, and has been paid more and more attention by researchers. Microwave heating mainly causes the shrinkage of carbon skeleton through rapid and efficient thermal action, which leads to the change of pore size and pore volume. In addition, the use of microwave heat treatment of activated carbon under different atmospheres will affect the properties of its surface groups, such as oxidizing atmosphere is conducive to the formation of acidic groups, reducing atmosphere is conducive to the formation of basic groups.
4.2 Surface oxidation method
 Under appropriate conditions, the surface of activated carbon is oxidized by using oxidants to remove some impurities on the surface and improve the content of oxygen-containing functional groups (such as carboxyl group, phenolic hydroxyl group, ester group, etc.) on the surface, which can improve the infiltration of carbon surface and also play a certain role in pore reaming. Oxidizing modification of the commonly used oxidants are nitric acid, hydrogen peroxide, sulfuric acid, ozone, ammonium persulfate, etc., the use of oxidants are different, the number and type of oxygen-containing functional groups of the products are different. In addition, the pore structure, specific surface area, volume and pore size of the modified activated carbon will also change. Nitric acid is the most commonly used strong oxidizing agent, and related studies have been widely reported. Dubi et al. used coconut shell activated carbon and apricot shell activated carbon as raw materials and used concentrated nitric acid surface modification to make electrodes for supercapacitors, and the discharge specific capacitance increased significantly. Ran Longguo et al. treated the activated carbon with nitric acid at different concentrations, and the specific surface area of the activated carbon after 10% nitric acid treatment was as high as that of the activated carbon
Under appropriate conditions, the surface of activated carbon is oxidized by using oxidants to remove some impurities on the surface and improve the content of oxygen-containing functional groups (such as carboxyl group, phenolic hydroxyl group, ester group, etc.) on the surface, which can improve the infiltration of carbon surface and also play a certain role in pore reaming. Oxidizing modification of the commonly used oxidants are nitric acid, hydrogen peroxide, sulfuric acid, ozone, ammonium persulfate, etc., the use of oxidants are different, the number and type of oxygen-containing functional groups of the products are different. In addition, the pore structure, specific surface area, volume and pore size of the modified activated carbon will also change. Nitric acid is the most commonly used strong oxidizing agent, and related studies have been widely reported. Dubi et al. used coconut shell activated carbon and apricot shell activated carbon as raw materials and used concentrated nitric acid surface modification to make electrodes for supercapacitors, and the discharge specific capacitance increased significantly. Ran Longguo et al. treated the activated carbon with nitric acid at different concentrations, and the specific surface area of the activated carbon after 10% nitric acid treatment was as high as that of the activated carbon
4.3 Surface reduction method
 Surface reduction modification refers to the reduction of functional groups on the surface of activated carbon with appropriate reducing agent at appropriate temperature, so as to increase the content of basic functional groups on the surface of activated carbon and enhance its non-polar surface, so as to improve its adsorption capacity for non-polar substances. The commonly used reducing agents are hydrogen, nitrogen, sodium hydroxide, potassium hydroxide and ammonia. Hydrogen or ammonia is the most commonly used method to prepare alkaline activated carbon. At 400-900 ℃, amides and aromatic amines can be generated on the surface of activated carbon after ammonia is added, and pyridine substances can be generated at higher temperatures. These nitrogenous functional groups will enhance the alkalinity of activated carbon surface. In addition, higher content of nitrogenous functional groups can be obtained by impregnating activated carbon in ammonia. Huang et al. found that the nitrogen content in the samples increased significantly after the modification of activated carbon by ammonia infiltration.
Surface reduction modification refers to the reduction of functional groups on the surface of activated carbon with appropriate reducing agent at appropriate temperature, so as to increase the content of basic functional groups on the surface of activated carbon and enhance its non-polar surface, so as to improve its adsorption capacity for non-polar substances. The commonly used reducing agents are hydrogen, nitrogen, sodium hydroxide, potassium hydroxide and ammonia. Hydrogen or ammonia is the most commonly used method to prepare alkaline activated carbon. At 400-900 ℃, amides and aromatic amines can be generated on the surface of activated carbon after ammonia is added, and pyridine substances can be generated at higher temperatures. These nitrogenous functional groups will enhance the alkalinity of activated carbon surface. In addition, higher content of nitrogenous functional groups can be obtained by impregnating activated carbon in ammonia. Huang et al. found that the nitrogen content in the samples increased significantly after the modification of activated carbon by ammonia infiltration.
4.4 Load atom method
 The supported atom modification method is to impregnate the activated carbon in a certain solution, make use of the huge specific surface area and pore volume of activated carbon, and introduce metal ions or other heteroatoms into the activated carbon pore by liquid deposition method, so as to increase the adsorption effect of activated carbon on the adsorbent. The metal ions commonly used for loading are copper ions, iron ions, aluminum ions and silver ions, etc. The activated carbon after loading shows good potential in adsorption of fluoride ions, cyanide and heavy metals and other pollutants
The supported atom modification method is to impregnate the activated carbon in a certain solution, make use of the huge specific surface area and pore volume of activated carbon, and introduce metal ions or other heteroatoms into the activated carbon pore by liquid deposition method, so as to increase the adsorption effect of activated carbon on the adsorbent. The metal ions commonly used for loading are copper ions, iron ions, aluminum ions and silver ions, etc. The activated carbon after loading shows good potential in adsorption of fluoride ions, cyanide and heavy metals and other pollutants
4.5 Low-temperature plasma method
 Plasma modification is a process of using non-cohesive plasma gas to modify the material surface. A plasma is an aggregate state of matter with a sufficient number of positive and negative charged particles of approximately equal charge. The low temperature plasma used for activated carbon modification is mainly produced by corona discharge, glow discharge and microwave discharge. The most commonly used is oxygen plasma, which has strong oxidation, when the plasma hits the surface of carbon materials, it can oxidize the crystal Angle, crystal edge and other defects or double bond structure into oxygen-containing functional groups. Modified by low-pressure oxygen and nitrogen plasma, activated carbon with a surface rich in nitro, amino and acyl amino groups can be obtained. Low temperature plasma modification technology is easy to operate, mild reaction conditions, low price, good environmental safety, and the treatment effect is only limited to the surface without affecting the properties of the material. In addition to the above modification methods, activated carbon modification methods also include surface acid-base modification method, ozone oxidation method, microwave radiation method, organic matter grafting method and so on. These methods have their own characteristics, and can be used alone or combined to modify activated carbon, so as to achieve better modification effect. In the process of use, suitable modification methods should be purposefully selected according to the properties of adsorbent.
Plasma modification is a process of using non-cohesive plasma gas to modify the material surface. A plasma is an aggregate state of matter with a sufficient number of positive and negative charged particles of approximately equal charge. The low temperature plasma used for activated carbon modification is mainly produced by corona discharge, glow discharge and microwave discharge. The most commonly used is oxygen plasma, which has strong oxidation, when the plasma hits the surface of carbon materials, it can oxidize the crystal Angle, crystal edge and other defects or double bond structure into oxygen-containing functional groups. Modified by low-pressure oxygen and nitrogen plasma, activated carbon with a surface rich in nitro, amino and acyl amino groups can be obtained. Low temperature plasma modification technology is easy to operate, mild reaction conditions, low price, good environmental safety, and the treatment effect is only limited to the surface without affecting the properties of the material. In addition to the above modification methods, activated carbon modification methods also include surface acid-base modification method, ozone oxidation method, microwave radiation method, organic matter grafting method and so on. These methods have their own characteristics, and can be used alone or combined to modify activated carbon, so as to achieve better modification effect. In the process of use, suitable modification methods should be purposefully selected according to the properties of adsorbent.
5 Application of activated carbon in supercapacitors-Graphene supercapacitor
 Activated carbon is the earliest carbon electrode material used in supercapacitors. Due to its abundant raw materials, low price and high specific surface area, it is still the first choice for commercial supercapacitors. Since Beck proposed to use activated carbon as electrode of double-layer capacitor in 1954, the application of activated carbon in supercapacitor has attracted much attention. According to the different source of raw carbon, the application of activated carbon in supercapacitors is briefly introduced.
Activated carbon is the earliest carbon electrode material used in supercapacitors. Due to its abundant raw materials, low price and high specific surface area, it is still the first choice for commercial supercapacitors. Since Beck proposed to use activated carbon as electrode of double-layer capacitor in 1954, the application of activated carbon in supercapacitor has attracted much attention. According to the different source of raw carbon, the application of activated carbon in supercapacitors is briefly introduced.
5.1 Fruit shell-based activated carbon
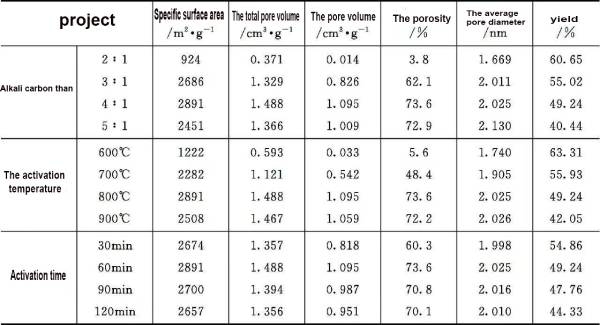
 Hou min, etc by the coconut shell charcoal material as raw material, KOH as activator, discussed the basic carbon ratio, activation temperature and activation time on the structure and properties of the activated carbon (table 2- 1), in carbon ratio, alkali activation temperature of 800 ℃, activation time, can prepare the specific surface area, total pore volume and pore diameter are mainly distributed in, porosity, average pore diameter of high quality activated carbon materials. The activated carbon is used as electrode material, the specific capacitance in the electrolyte can be reached, has excellent electrochemical performance; Yang Jing et al. with walnut shell as raw material, using the secondary activation method to prepare activated carbon, charge/discharge, mass ratio capacitance as high as, capacitor energy density as high. Chen Xiaomei et al. prepared activated carbon electrode material by chemical activation method with walnut shell as precursor and KOH as electrolyte The test results show that the prepared activated carbon electrode material has ideal electrochemical capacitance behavior, the specific capacitance is as high as 0.25mA, the leakage current and the equivalent series resistance are only 0.39Ω, respectively. After 5000 cycles of charging/discharging, the capacitance still remains above 88%.
Hou min, etc by the coconut shell charcoal material as raw material, KOH as activator, discussed the basic carbon ratio, activation temperature and activation time on the structure and properties of the activated carbon (table 2- 1), in carbon ratio, alkali activation temperature of 800 ℃, activation time, can prepare the specific surface area, total pore volume and pore diameter are mainly distributed in, porosity, average pore diameter of high quality activated carbon materials. The activated carbon is used as electrode material, the specific capacitance in the electrolyte can be reached, has excellent electrochemical performance; Yang Jing et al. with walnut shell as raw material, using the secondary activation method to prepare activated carbon, charge/discharge, mass ratio capacitance as high as, capacitor energy density as high. Chen Xiaomei et al. prepared activated carbon electrode material by chemical activation method with walnut shell as precursor and KOH as electrolyte The test results show that the prepared activated carbon electrode material has ideal electrochemical capacitance behavior, the specific capacitance is as high as 0.25mA, the leakage current and the equivalent series resistance are only 0.39Ω, respectively. After 5000 cycles of charging/discharging, the capacitance still remains above 88%.
5.2 Rice husk-based activated carbon
 Song Xiaolan et al. used rice husk as raw material and NaOH as activator to obtain activated carbon with specific surface area at 800℃. In KOH electrolyte, the specific capacitance of capacitor was up to, and after 5000 cycles, the capacitance retention rate was still there. Using rice husk as raw material and NaOH as activator, He et al. obtained activated carbon with specific surface area as microwave heating, and its specific capacitance was still up to after the second cycle. After carbonizing rice husk under nitrogen atmosphere, impregnating with HF and activating with KOH, the activated carbon with graded pore structure containing large mesopore and rich micropore was finally obtained, with a high specific surface area and energy density
Song Xiaolan et al. used rice husk as raw material and NaOH as activator to obtain activated carbon with specific surface area at 800℃. In KOH electrolyte, the specific capacitance of capacitor was up to, and after 5000 cycles, the capacitance retention rate was still there. Using rice husk as raw material and NaOH as activator, He et al. obtained activated carbon with specific surface area as microwave heating, and its specific capacitance was still up to after the second cycle. After carbonizing rice husk under nitrogen atmosphere, impregnating with HF and activating with KOH, the activated carbon with graded pore structure containing large mesopore and rich micropore was finally obtained, with a high specific surface area and energy density
5.3 Bamboo charcoal based activated carbon
 Bamboo as a kind of renewable biomass raw material has the characteristics of fast growth, rapid renewal and strong regeneration ability, and bamboo resources are rich and widely distributed in our country, which provide convenience for us on raw materials. Bamboo charcoal is a solid product obtained by carbonization of bamboo at high temperature and without oxygen. It has the characteristics of high mechanical strength, developed pore structure, large specific surface area and good electrical conductivity. Lee et al. found that bamboo activated carbon has good micropore structure and high mesoporous rate, and can significantly improve the permeability of electrolyte when used as a fast ion channel in the electrode of supercapacitor. Bai Xiang et al. with bamboo as raw material, the carbonization of bamboo charcoal first physical activation, and then in the activation, the specific surface area of bamboo charcoal based activated carbon, pore size distribution between, specific capacity up to, and showed a good high current charge/discharge performance. Yang Shengjie et al. prepared activated carbon at high temperature under the protection of inert atmosphere with Moso bamboo as raw material, sodium hydroxide as activator, and obtained the optimal process conditions: activation time 2h, activation temperature, alkalo-carbon ratio (mass ratio), the first discharge ratio capacitance of the prepared material, the second cycle ratio capacitance retention rate, and the leakage current of the prepared material
Bamboo as a kind of renewable biomass raw material has the characteristics of fast growth, rapid renewal and strong regeneration ability, and bamboo resources are rich and widely distributed in our country, which provide convenience for us on raw materials. Bamboo charcoal is a solid product obtained by carbonization of bamboo at high temperature and without oxygen. It has the characteristics of high mechanical strength, developed pore structure, large specific surface area and good electrical conductivity. Lee et al. found that bamboo activated carbon has good micropore structure and high mesoporous rate, and can significantly improve the permeability of electrolyte when used as a fast ion channel in the electrode of supercapacitor. Bai Xiang et al. with bamboo as raw material, the carbonization of bamboo charcoal first physical activation, and then in the activation, the specific surface area of bamboo charcoal based activated carbon, pore size distribution between, specific capacity up to, and showed a good high current charge/discharge performance. Yang Shengjie et al. prepared activated carbon at high temperature under the protection of inert atmosphere with Moso bamboo as raw material, sodium hydroxide as activator, and obtained the optimal process conditions: activation time 2h, activation temperature, alkalo-carbon ratio (mass ratio), the first discharge ratio capacitance of the prepared material, the second cycle ratio capacitance retention rate, and the leakage current of the prepared material
5.4 Coal-based activated carbon
 Coal based activated carbon yield accounts for more than 70% of total activated carbon production in China, is the main variety of activated carbon production in China. Coal-based activated carbon has the characteristics of low production cost, wide source, simple preparation method, good electrical conductivity, high specific surface area, strong corrosion resistance, controllable pore structure and stable electrochemical performance. Therefore, coal-based activated carbon as electrode material has broad application prospects. Zhang Chuanxiang et al. Taixi anthracite as raw material, as activator, under the condition of carboniferous ratio, activation of activated carbon for specific surface area, mesoporous rate is, in the electrolyte capacitance is as high as, and under the high current density charge/discharge ratio capacitance retention rate is high, leakage current is only. Xing Baolin et al Henan Yongcheng anthracite as raw material, coal based activated carbon with high specific surface area, the capacitance is as high as, after the cycle, the ratio of capacitance to maintain the rate. Using bituminous coal as raw material, Zhang et al. prepared a kind of oxygen-rich activated carbon with medium specific surface area by rapid activation method. The oxygen-rich activated carbon as electrode material has higher energy density and power density, and its specific capacitance is as high as and at and current densities, respectively.
Coal based activated carbon yield accounts for more than 70% of total activated carbon production in China, is the main variety of activated carbon production in China. Coal-based activated carbon has the characteristics of low production cost, wide source, simple preparation method, good electrical conductivity, high specific surface area, strong corrosion resistance, controllable pore structure and stable electrochemical performance. Therefore, coal-based activated carbon as electrode material has broad application prospects. Zhang Chuanxiang et al. Taixi anthracite as raw material, as activator, under the condition of carboniferous ratio, activation of activated carbon for specific surface area, mesoporous rate is, in the electrolyte capacitance is as high as, and under the high current density charge/discharge ratio capacitance retention rate is high, leakage current is only. Xing Baolin et al Henan Yongcheng anthracite as raw material, coal based activated carbon with high specific surface area, the capacitance is as high as, after the cycle, the ratio of capacitance to maintain the rate. Using bituminous coal as raw material, Zhang et al. prepared a kind of oxygen-rich activated carbon with medium specific surface area by rapid activation method. The oxygen-rich activated carbon as electrode material has higher energy density and power density, and its specific capacitance is as high as and at and current densities, respectively.
5.5 Petroleum-based activated carbon
 Petroleum coke is a by-product of petrochemical refining industry, which has abundant resources, wide distribution, low price, high carbon content, high yield of activated carbon and large specific surface area. In addition, the ash and volatile matter of petroleum coke are low, and the activated carbon produced has low impurity content and excellent performance. Song Yan et al. investigated the effects of different influencing factors on the properties of activated carbon products through orthogonal experiments, and the order of the effects was as follows: microstructure of raw coke > activation temperature > alkali-carbon ratio > particle size of raw material > activation time (” > “means” better “). Under the optimal combination conditions, Panjin petroleum coke was used as raw material and activated by KOH to prepare activated carbon with specific surface area. At the same time, it was found that the coke with small grain and fine Mosaic optical structure in the raw material had the highest reactivity with the activator, and the prepared activated carbon had the largest specific surface area. Tan Minghui et al. obtained porous carbon with petroleum coke as raw material and KOH activation, impregnated it in ferric nitrate solution after carbonization and high temperature activation of carbon dioxide, and prepared activated carbon with specific surface area and total pore volume. After the second activation by metal salt impregnation, the mesoporous ratio of the porous carbon increases from to, which significantly increases the charging/discharging rate of the electrode. By introducing the active gas hydrogen, Xiao Ronglin et al. obtained activated carbon with high specific surface area under the condition of reducing the amount of alkali, and showed better performance than the activated carbon prepared without introducing hydrogen. The added hydrogen can react with the functional groups on the surface of activated carbon, provide more active points, promote the development of the pore structure of activated carbon, and adjust the distribution of pore structure.
Petroleum coke is a by-product of petrochemical refining industry, which has abundant resources, wide distribution, low price, high carbon content, high yield of activated carbon and large specific surface area. In addition, the ash and volatile matter of petroleum coke are low, and the activated carbon produced has low impurity content and excellent performance. Song Yan et al. investigated the effects of different influencing factors on the properties of activated carbon products through orthogonal experiments, and the order of the effects was as follows: microstructure of raw coke > activation temperature > alkali-carbon ratio > particle size of raw material > activation time (” > “means” better “). Under the optimal combination conditions, Panjin petroleum coke was used as raw material and activated by KOH to prepare activated carbon with specific surface area. At the same time, it was found that the coke with small grain and fine Mosaic optical structure in the raw material had the highest reactivity with the activator, and the prepared activated carbon had the largest specific surface area. Tan Minghui et al. obtained porous carbon with petroleum coke as raw material and KOH activation, impregnated it in ferric nitrate solution after carbonization and high temperature activation of carbon dioxide, and prepared activated carbon with specific surface area and total pore volume. After the second activation by metal salt impregnation, the mesoporous ratio of the porous carbon increases from to, which significantly increases the charging/discharging rate of the electrode. By introducing the active gas hydrogen, Xiao Ronglin et al. obtained activated carbon with high specific surface area under the condition of reducing the amount of alkali, and showed better performance than the activated carbon prepared without introducing hydrogen. The added hydrogen can react with the functional groups on the surface of activated carbon, provide more active points, promote the development of the pore structure of activated carbon, and adjust the distribution of pore structure.
5.6 Bitum-based activated carbon
 Asphalt is a complex mixture of dark brown and high viscosity composed of different molecular weight hydrocarbon compounds and their nonmetallic derivatives, which can be mainly divided into coal tar asphalt, petroleum asphalt and natural asphalt. Among them, coal tar pitch is a by-product of coking, which has the advantages of not being disturbed by season, low price and high carbonization yield, and has been used by a large number of researchers as electrode materials for supercapacitors. Using magnesium oxide as template and coal tar pitch as carbon source, the mesoporous activated carbon material was prepared by one-step heating. The material has high specific capacitance, and the specific capacitance under and current density is and respectively. Using carbonized meso-phase asphalt as precursor and activator, Jing Ulrich prepared activated carbon electrode materials for supercapacitors. The effects of activation temperature, alkali-carbon ratio and process conditions on the pore structure and electrochemical behavior of activated carbon were investigated. The results show that the maximum specific capacitance of the activated carbon electrode prepared under the conditions of activation temperature and alkali-carbon ratio can be reached at time, the changes of the pore structure and specific capacitance of the activated carbon depend on the specific treatment process, and the mesoporous content has an important influence on the specific capacitance of the activated carbon electrode. He et al.] took coal tar pitch as raw material, only a small amount of KOH activator was used, and heated under the assistance of microwave to obtain activated carbon with a specific surface area. The performance of supercapacitor electrode material in different electrolytes is studied. It is found that the ratio of capacitor in the electrolyte is higher than that in the capacitor, and the energy density in the solution is higher.
Asphalt is a complex mixture of dark brown and high viscosity composed of different molecular weight hydrocarbon compounds and their nonmetallic derivatives, which can be mainly divided into coal tar asphalt, petroleum asphalt and natural asphalt. Among them, coal tar pitch is a by-product of coking, which has the advantages of not being disturbed by season, low price and high carbonization yield, and has been used by a large number of researchers as electrode materials for supercapacitors. Using magnesium oxide as template and coal tar pitch as carbon source, the mesoporous activated carbon material was prepared by one-step heating. The material has high specific capacitance, and the specific capacitance under and current density is and respectively. Using carbonized meso-phase asphalt as precursor and activator, Jing Ulrich prepared activated carbon electrode materials for supercapacitors. The effects of activation temperature, alkali-carbon ratio and process conditions on the pore structure and electrochemical behavior of activated carbon were investigated. The results show that the maximum specific capacitance of the activated carbon electrode prepared under the conditions of activation temperature and alkali-carbon ratio can be reached at time, the changes of the pore structure and specific capacitance of the activated carbon depend on the specific treatment process, and the mesoporous content has an important influence on the specific capacitance of the activated carbon electrode. He et al.] took coal tar pitch as raw material, only a small amount of KOH activator was used, and heated under the assistance of microwave to obtain activated carbon with a specific surface area. The performance of supercapacitor electrode material in different electrolytes is studied. It is found that the ratio of capacitor in the electrolyte is higher than that in the capacitor, and the energy density in the solution is higher.
5.7 Phenolic resin-based activated carbon
 Phenolic resin is obtained by polycondensation of phenolic compounds and aldehydes, among which the resin obtained by polycondensation of phenol and formaldehyde is the most important. As the earliest synthetic polymer, phenolic resin has attracted more and more attention because of its mature production process, low price, high carbonization yield, easy activation of pores and large specific surface area. Teng et al. used phenolic resin as raw material and as activator to prepare activated carbon with specific surface area and medium specific capacity. Geng Xin et al. prepared activated carbon with high specific surface area for supercapacitors by activation method using water-soluble phenolic resin as raw material. The results show that the activated carbon prepared at 650℃ has the largest specific surface area and the smallest micropore ratio. However, the micropores of activated carbon prepared at 700℃ and 750℃ are interconnected, and the conductivity and ion migration resistance are better than those of the former. Using silica sol as template and phenolic resin as carbon source, Wang Renqing et al. prepared activated carbon with specific surface area by template method. Under the current density, the specific capacitance could be reached. In addition, the pore size and pore size distribution of the product carbon can be controlled by adding materials such as polyvinyl alcohol or polyvinyl butyral, which are easy to crack and have low carbon residue, into the phenolic resin.
Phenolic resin is obtained by polycondensation of phenolic compounds and aldehydes, among which the resin obtained by polycondensation of phenol and formaldehyde is the most important. As the earliest synthetic polymer, phenolic resin has attracted more and more attention because of its mature production process, low price, high carbonization yield, easy activation of pores and large specific surface area. Teng et al. used phenolic resin as raw material and as activator to prepare activated carbon with specific surface area and medium specific capacity. Geng Xin et al. prepared activated carbon with high specific surface area for supercapacitors by activation method using water-soluble phenolic resin as raw material. The results show that the activated carbon prepared at 650℃ has the largest specific surface area and the smallest micropore ratio. However, the micropores of activated carbon prepared at 700℃ and 750℃ are interconnected, and the conductivity and ion migration resistance are better than those of the former. Using silica sol as template and phenolic resin as carbon source, Wang Renqing et al. prepared activated carbon with specific surface area by template method. Under the current density, the specific capacitance could be reached. In addition, the pore size and pore size distribution of the product carbon can be controlled by adding materials such as polyvinyl alcohol or polyvinyl butyral, which are easy to crack and have low carbon residue, into the phenolic resin.