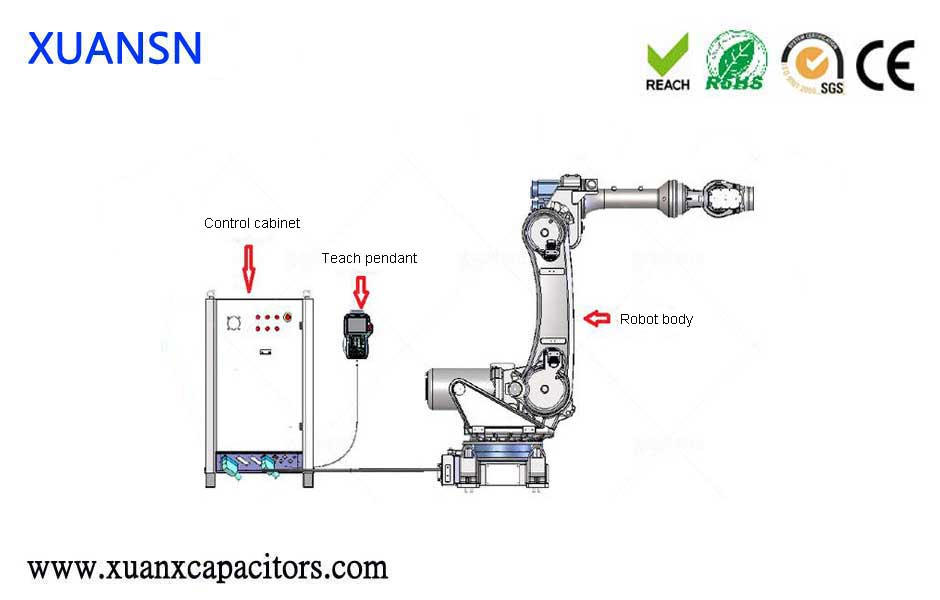
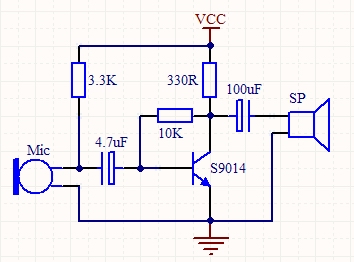
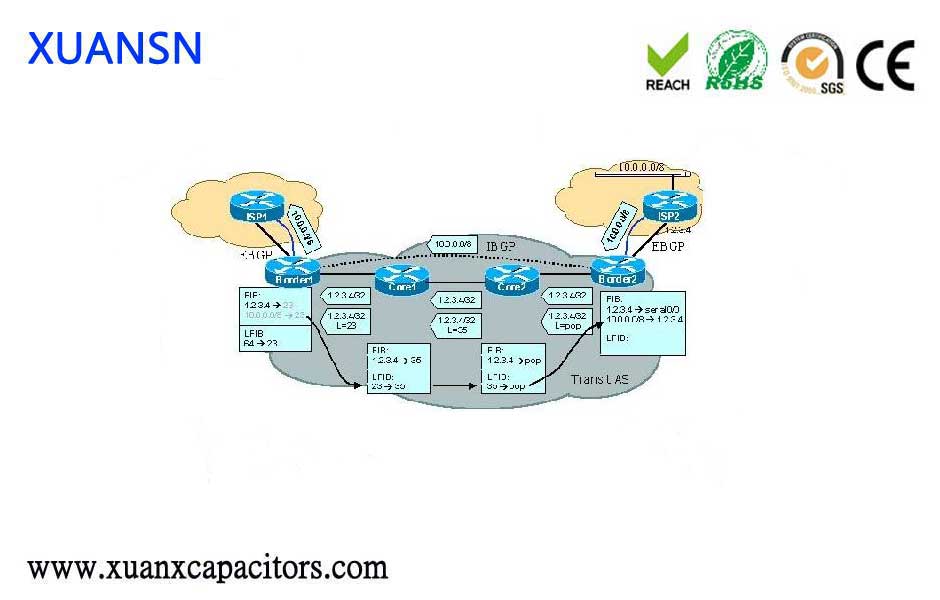
It is the only COVID 19 vaccine in Phase II clinical trials in the world, according to the WHO website.
 Compared with Phase I, the Phase II trial of the COVID 19 vaccine lowered the age limit and increased the number of elderly volunteers over 60. The vaccine must provide a safety barrier to the more senior citizens in COVID 19. In the process of prevention and control of the COVID 19 epidemic, the Chinese organization has carried out a variety of technical routes of vaccine research and development, including adenoviral vector recombinant COVID 19 vaccine research and development work has made significant progress, is currently in the international leading position. The vaccine takes the modified replication-defective Adenovirus as the carrier, carries the s gene of the new coronavirus, and enters the subjects to make the human body produce the immune memory of the s protein, thus achieving the effect of “shutting out” the virus. Hopefully more good news from COVID 19 will follow.
Compared with Phase I, the Phase II trial of the COVID 19 vaccine lowered the age limit and increased the number of elderly volunteers over 60. The vaccine must provide a safety barrier to the more senior citizens in COVID 19. In the process of prevention and control of the COVID 19 epidemic, the Chinese organization has carried out a variety of technical routes of vaccine research and development, including adenoviral vector recombinant COVID 19 vaccine research and development work has made significant progress, is currently in the international leading position. The vaccine takes the modified replication-defective Adenovirus as the carrier, carries the s gene of the new coronavirus, and enters the subjects to make the human body produce the immune memory of the s protein, thus achieving the effect of “shutting out” the virus. Hopefully more good news from COVID 19 will follow.